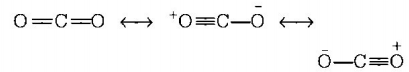1. Which of the following molecules does not show any resonating structures?
A
$$N{H_3}$$
B
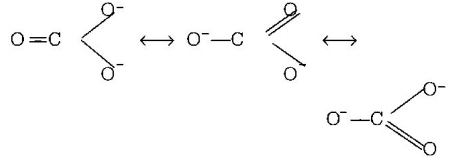
$$CO_3^{2 - }$$
C
$${O_3}$$
D
$$S{O_3}$$
Answer :
$$N{H_3}$$
2. Which of the following substances has the least ionic character ?
A
$$FeC{l_2}$$
B
$$ZnC{l_2}$$
C
$$CdC{l_2}$$
D
$$MgC{l_2}$$
Answer :
$$ZnC{l_2}$$
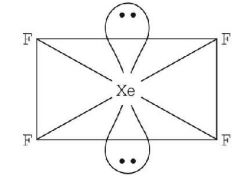
3. Which of the following has the square planar structure?
A
$$Xe{F_4}$$
B
$$NH_4^ + $$
C
$$BF_4^ - $$
D
$$CC{l_4}$$
Answer :
$$Xe{F_4}$$
4. Amongst $$LiCl,RbCl,BeC{l_2}$$ and $$MgC{l_2}$$ the compounds with the greatest and the least ionic character, respectively are:
A
$$LiCl\,{\text{and}}\,RbCl$$
B
$$RbCl\,{\text{and}}\,BeC{l_2}$$
C
$$MgC{l_2}\,{\text{and}}\,BeC{l_2}$$
D
$$RbCl\,{\text{and}}\,MgC{l_2}$$
Answer :
$$RbCl\,{\text{and}}\,BeC{l_2}$$
5. The octet rule is not valid for the molecule
A
$$C{O_2}$$
B
$${H_2}O$$
C
$${O_2}$$
D
$$CO$$
Answer :
$$CO$$
6. Which one of the following is planar?
A
$$Xe{F_4}$$
B
$$Xe{O_4}$$
C
$$Xe{O_3}F$$
D
$$Xe{O_3}{F_2}$$
Answer :
$$Xe{F_4}$$
7. The correct order of $$C - O$$ bond length among $$CO,CO_3^{2 - },C{O_2}$$ is
A
$$C{O_2} < CO_3^{2 - } < CO$$
B
$$CO < CO_3^{2 - } < C{O_2}$$
C
$$CO_3^{2 - } < C{O_2} < CO$$
D
$$CO < C{O_2} < CO_3^{2 - }$$
Answer :
$$CO < C{O_2} < CO_3^{2 - }$$
8. Equilateral shape has
A
$$sp$$ hybridisation
B
$$s{p^2}$$ hybridisation
C
$$s{p^3}$$ hybridisation
D
$$ds{p^2}$$ hybridisation
Answer :
$$s{p^2}$$ hybridisation
9.
Which of the following observations can be explained on the basis of hydrogen bonding?
(i) $$H-F$$ has higher boiling point than other halogen acids.
(ii) $${H_2}O$$ has highest boiling point among hydrides of group 16 elements.
(iii) $$N{H_3}$$ has lower boiling point than $$P{H_3}.$$
A
(i), (ii) and (iii)
B
(i) and (iii)
C
(ii) and (iii)
D
(i) and (ii)
Answer :
(i) and (ii)
10. Isostructural species are those which have the same shape and hybridisation. Among the given species, identify the isostructural pairs.
A
$$\left[ {N{F_3}\,{\text{and}}\,B{F_3}} \right]$$
B
$$\left[ {BF_4^ - \,{\text{and}}\,NH_4^ + } \right]$$
C
$$\left[ {BC{l_3}\,{\text{and}}\,BrC{l_3}} \right]$$
D
$$\left[ {N{H_3}\,{\text{and}}\,NO_3^ - } \right]$$
Answer :
$$\left[ {BF_4^ - \,{\text{and}}\,NH_4^ + } \right]$$