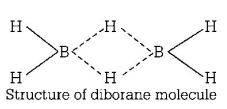
111. The type of hybridisation of boron in diborane is
A
$$sp$$ hybridisation
B
$$s{p^2}$$ hybridisation
C
$$s{p^3}$$ hybridisation
D
$$s{p^3}{d^2}$$ hybridisation
Answer :
$$s{p^3}$$ hybridisation
112. Linear combination of two hybridised orbitals belonging to the two atoms, each having one electron leads to a
A
sigma bond
B
double bond
C
coordinate bond
D
pi-bond
Answer :
sigma bond
113. Propyne molecule contains
A
6 $$sigma$$ and 2 $$pi$$ bonds
B
5 $$sigma$$ bonds
C
5 $$pi$$ bonds and 1 $$sigma$$ bond
D
2 $$sigma$$ and 3 $$pi$$ bonds
Answer :
6 $$sigma$$ and 2 $$pi$$ bonds
114.
The radius and charge of each of six ions are shown in the table :
| Ion | $${J^ + }$$ | $${L^ + }$$ | $${M^{2 + }}$$ | $${X^ - }$$ | $${Y^ - }$$ | $${Z^{2 - }}$$ |
|---|---|---|---|---|---|---|
| Radius/$$nm$$ | 0.14 | 0.18 | 0.15 | 0.14 | 0.18 | 0.15 |
The ionic solids $$JX,LY$$ and $$MZ$$ are of the same lattice type. What is the correct order of their lattice energies placing the one with the highest numerical value first?
A
$$JX > LY > MZ$$
B
$$JX > MZ > LY$$
C
$$LY > MZ > JX$$
D
$$MZ > JX > LY$$
Answer :
$$MZ > JX > LY$$
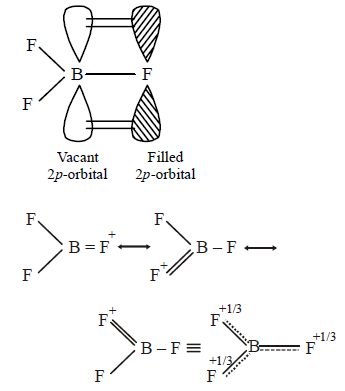
115. The bond dissociation energy of $$B-F$$ in $$B{F_3}$$ is $$646\,kJ\,mo{l^{ - 1}}$$ whereas that of $$C-F$$ in $$C{F_4}$$ is $$515\,kJ\,mo{l^{ - 1}}.$$ The correct reason for higher $$B-F$$ bond dissociation energy as compared to that of$$C-F$$ is
A
stronger $$\sigma $$ bond between $$B$$ and $$F$$ in $$B{F_3}$$ as compared to that between $$C$$ and $$F$$ in $$C{F_4}.$$
B
significant $$p\pi - p\pi $$ interaction between $$B$$ and $$F$$ in $$B{F_3}$$ whereas there is no possibility of such interaction between $$C$$ and $$F$$ in $$C{F_4}.$$
C
lower degree of $$p\pi - p\pi $$ interaction between $$B$$ and $$F$$ in $$B{F_3}$$ than that between $$C$$ and $$F$$ in $$C{F_4}.$$
D
Smaller size of $$B$$-atom as compared to that of $$C$$-atom.
Answer :
significant $$p\pi - p\pi $$ interaction between $$B$$ and $$F$$ in $$B{F_3}$$ whereas there is no possibility of such interaction between $$C$$ and $$F$$ in $$C{F_4}.$$
116. During a coordinate bond formation,
A
one electron from an atom is transferred to other
B
one electron each is lost from both the atoms
C
a pair of electrons is contributed by one atom and shared by both the atoms
D
a pair of electrons is transferred to the other atom
Answer :
a pair of electrons is contributed by one atom and shared by both the atoms
117. Among the following the exceptions of the octet rule is
A
the incomplete octet of central atom
B
an odd number of electrons on central atom
C
expanded octet of the central atom
D
all of these
Answer :
all of these
118. The correct order of bond dissociation energy among $${N_2},{O_2},O_2^ - $$ is shown in which of the following arrangements?
A
$${N_2} > O_2^ - > {O_2}$$
B
$$O_2^ - > {O_2} > {N_2}$$
C
$${N_2} > {O_2} > O_2^ - $$
D
$${O_2} > O_2^ - > {N_2}$$
Answer :
$${N_2} > {O_2} > O_2^ - $$
119. In $$PO_4^{3 - }$$ the formal charge on each $$O$$ - atom and $$P - O$$ bond order respectively are
A
- 0.75, 1.0
B
- 0.75, 1.25
C
- 0.75, 0.6
D
- 3, 1.25
Answer :
- 0.75, 1.25
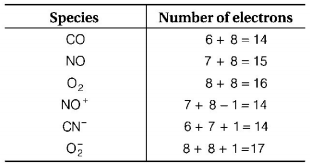
120. Which one of the following pairs of species have the same bond order?
A
$$CO,NO$$
B
$${O_2},N{O^ + }$$
C
$$C{N^ - },CO$$
D
$${N_2},O_2^ - $$
Answer :
$$C{N^ - },CO$$