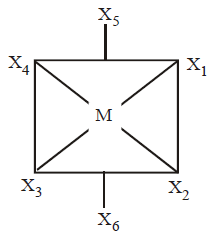131. Number of bond pairs and lone pairs around the central atom in $$I_3^ - $$ ion, respectively are
A
2, 2
B
2, 3
C
3, 2
D
4, 3
Answer :
2, 3
132. In a regular octahedral molecule, $$M{X_6}$$ the number of $$X - M - X$$ bonds at $${180^ \circ }$$ is
A
three
B
two
C
six
D
four
Answer :
three
133. Bond order of 1.5 is shown by
A
$$O_2^ + $$
B
$$O_2^ - $$
C
$$O_2^{2 - }$$
D
$${O_2}$$
Answer :
$$O_2^ - $$
134. Which of the following compounds are covalent?
A
$${H_2}$$
B
$$CaO$$
C
$$KCl$$
D
$$N{a_2}S$$
Answer :
$${H_2}$$
135. Which one of the following molecules is polar ?
A
$$Xe{F_4}$$
B
$$I{F_5}$$
C
$$Sb{F_5}$$
D
$$C{F_4}$$
Answer :
$$I{F_5}$$
136. How many number of electrons are involved in the formation of a nitrogen molecule?
A
Three
B
Four
C
Eight
D
Six
Answer :
Six
137. In which of the following pairs, the two species are isostructural?
A
$$S{F_4}\,{\text{and}}\,Xe{F_4}$$
B
$$SO_3^{2 - }\,{\text{and}}\,NO_3^ - $$
C
$$B{F_3}\,\,{\text{and}}\,N{F_3}$$
D
$$BrO_3^ - \,{\text{and}}\,Xe{O_3}$$
Answer :
$$BrO_3^ - \,{\text{and}}\,Xe{O_3}$$
138. Among the following group which represents the collection of isoelectronic species?
A
$$NO,C{N^ - },{N_2},O_2^ - $$
B
$$N{O^ + },C_2^{2 - },O_2^ - ,CO$$
C
$${N_2},C_2^{2 - },CO,NO$$
D
$$CO,N{O^ + },C{N^ - },C_2^{2 - }$$
Answer :
$$CO,N{O^ + },C{N^ - },C_2^{2 - }$$
139. The species in which the $$N$$ atom is in a state of $$sp$$ hybridization is :
A
$$NO_3^ - $$
B
$$N{O_2}$$
C
$$NO_2^ + $$
D
$$NO_2^ - $$
Answer :
$$NO_2^ + $$
140.
The increasing order of energies of various molecular orbitals of $${N_2}$$ is given below :
$$\sigma 1s < {\sigma ^ * }1s < \sigma 2s < {\sigma ^ * }2s < \pi 2{p_x}$$ $$ = \pi 2{p_y} < \sigma 2{p_z} < {\pi ^ * }2{p_x}$$ $$ = {\pi ^ * }2{p_y} < {\sigma ^ * }2{p_z}$$
The above sequence is not true for the molecule
A
$${C_2}$$
B
$${B_2}$$
C
$${O_2}$$
D
$$B{e_2}$$
Answer :
$${O_2}$$