61. Which of the following order of energies of molecular orbitals of $${N_2}$$ is correct?
A
$$\left( {\pi 2{p_y}} \right) < \left( {\sigma 2{p_z}} \right) < \left( {{\pi ^ * }2{p_x}} \right)$$ $$ = \left( {{\pi ^ * }2{p_y}} \right)$$
B
$$\left( {\pi 2{p_y}} \right) > \left( {\sigma 2{p_z}} \right) > \left( {{\pi ^ * }2{p_x}} \right)$$ $$ = \left( {{\pi ^ * }2{p_y}} \right)$$
C
$$\left( {\pi 2{p_y}} \right) < \left( {\sigma 2{p_z}} \right) > \left( {{\pi ^ * }2{p_x}} \right)$$ $$ = \left( {{\pi ^ * }2{p_y}} \right)$$
D
$$\left( {\pi 2{p_y}} \right) > \left( {\sigma 2{p_z}} \right) < \left( {{\pi ^ * }2{p_x}} \right)$$ $$ = \left( {{\pi ^ * }2{p_y}} \right)$$
Answer :
$$\left( {\pi 2{p_y}} \right) < \left( {\sigma 2{p_z}} \right) < \left( {{\pi ^ * }2{p_x}} \right)$$ $$ = \left( {{\pi ^ * }2{p_y}} \right)$$
62. According to molecular orbital theory, which of the following is true with respect to $$Li_2^ + $$ and $$Li_2^ - ?$$
A
\[Li_2^ + \,\] is unstable and \[Li_2^ - \,\] is stable
B
\[Li_2^ + \] is stable and \[Li_2^ - \] is unstable
C
Both are stable
D
Both are unstable
Answer :
Both are stable
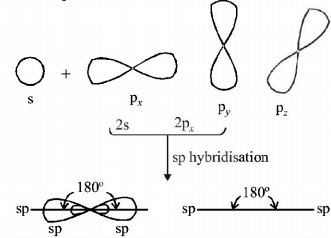
63. On hybridization of one $$s$$ and one $$p$$ orbitals we get :
A
two mutually perpendicular orbitals
B
two orbitals at $${180^ \circ }$$
C
four orbitals directed tetrahedrally
D
three orbitals in a plane
Answer :
two orbitals at $${180^ \circ }$$
64.
Consider the following molecules
$$\mathop {{O_2}}\limits_{\text{I}} ,\mathop {{O_2}\left( {As{F_6}} \right)}\limits_{{\text{II}}} ,\mathop {K{O_2}}\limits_{{\text{III}}} $$
Choose the correct answer.
A
The correct decreasing bond order is II > I > III.
B
The correct decreasing order of bond length is III > II > I.
C
The bond strength of I is less than that of III.
D
Bond dissociation energy is highest in case of III.
Answer :
The correct decreasing bond order is II > I > III.
65. Which one of the following pairs of species have the same bond order?
A
$$C{N^ - }\,\,{\text{and}}\,\,{\text{N}}{{\text{O}}^ + }$$
B
$$C{N^ - }\,\,{\text{and}}\,\,C{N^ + }$$
C
$$O_2^ - \,\,{\text{and}}\,\,C{N^ - }$$
D
$$N{O^ + }\,\,\,{\text{and}}\,\,C{N^ + }\,\,$$
Answer :
$$C{N^ - }\,\,{\text{and}}\,\,{\text{N}}{{\text{O}}^ + }$$
66. What is common between the following molecules $$S{O_3},CO_3^{2 - },NO_3^ - ?$$
A
All have linear shape.
B
All have trigonal planar shape.
C
All have tetrahedral shape.
D
All have trigonal pyramidal shape.
Answer :
All have trigonal planar shape.
67. Which of the following species is not paramagnetic ?
A
$$NO$$
B
$$CO$$
C
$${O_2}$$
D
$${B_2}$$
Answer :
$$CO$$
68. Although $$C{N^ - }\,ion$$ and $${N_2}$$ molecule are isoelectronic, yet $${N_2}$$ molecule is chemically inert because of
A
presence of more number of electrons in bonding orbitals
B
lone bond energy
C
absence of bond polarity
D
uneven electron distribution.
Answer :
absence of bond polarity
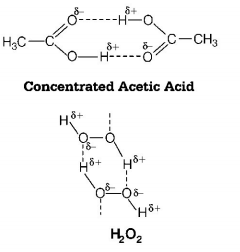
69. Which one of the following compounds shows the presence of intramolecular hydrogen bond?
A
$${H_2}{O_2}$$
B
$$HCN$$
C
$${\text{Cellulose}}$$
D
$${\text{Concentrated acetic acid}}$$
Answer :
$${\text{Cellulose}}$$
70. Amongst the following elements whose electronic configurations are given below, the one having the highest ionisation enthalpy is
A
$$\left[ {Ne} \right]3{s^2}3{p^1}$$
B
$$\left[ {Ne} \right]3{s^2}3{p^3}$$
C
$$\left[ {Ne} \right]3{s^2}3{p^2}$$
D
$$\left[ {Ar} \right]3{d^{10}}4{s^2}4{p^3}$$
Answer :
$$\left[ {Ne} \right]3{s^2}3{p^3}$$