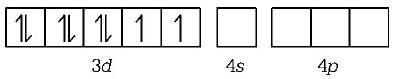
231. Considering $${H_2}O$$ as a weak field ligand, the number of unpaired electrons in $${\left[ {Mn{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$ will be ( At. no. of $$Mn = 25$$ )
A
3
B
5
C
2
D
4
Answer :
5
232. A magnetic moment of $$1.73\,BM$$ will be shown by one among the following
A
$${\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}$$
B
$${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$$
C
$$TiC{l_4}$$
D
$${\left[ {CoC{l_6}} \right]^{4 - }}$$
Answer :
$${\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}$$
233. Hydrogen peroxide oxidises $${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}$$ to $${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{3 - }}$$ in acidic medium but reduces $${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{3 - }}$$ to $${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}\,$$ in alkaline medium. The other products formed are respectively:
A
$$\left( {{H_2}O + {O_2}} \right)\,{\text{and}}\,{H_2}O$$
B
$$\left( {{H_2}O + {O_2}} \right)\,{\text{and}}\,\left( {{H_2}O + O{H^ - }} \right)$$
C
$${H_2}O\,\,{\text{and}}\,\left( {{H_2}O + {O_2}} \right)$$
D
$${H_2}O\,\,{\text{and}}\,\left( {{H_2}O + O{H^ - }} \right)$$
Answer :
$${H_2}O\,\,{\text{and}}\,\left( {{H_2}O + {O_2}} \right)$$
234. For octahedral complex, which of the following $${d^n}$$ configurations of metal cation cannot exist in high spin and low spin forms ?
A
$${d^3}{\text{ and }}{d^8}$$
B
$${d^3}{\text{ and }}{d^5}$$
C
$${d^3}{\text{ and }}{d^6}$$
D
$${d^4}{\text{ and }}{d^8}$$
Answer :
$${d^3}{\text{ and }}{d^8}$$
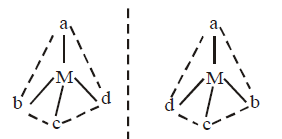
235. The terahedral complex $$\left[ {M\left( A \right)\left( B \right)\left( X \right)\left( Y \right)} \right],$$ where $$A,B,X$$ and $$Y$$ are different ligands and $$M$$ is a metal ion is
A
optically inactive
B
rotate plane polarized light
C
incomplete information
D
can’t be said
Answer :
rotate plane polarized light
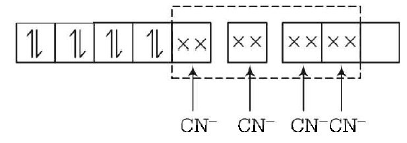
236. Of the following complex ions which is diamagnetic in nature ?
A
$${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$$
B
$${\left[ {CuC{l_4}} \right]^{2 - }}$$
C
$${\left[ {Co{F_6}} \right]^{3 - }}$$
D
$${\left[ {NiC{l_4}} \right]^{2 - }}$$
Answer :
$${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$$
237. Possible isomerism in complexes $$\left[ {Co{{\left( {N{H_3}} \right)}_3}{{\left( {N{O_2}} \right)}_3}} \right]$$ and $$\left[ {Co{{\left( {N{H_3}} \right)}_5}\left( {N{O_2}} \right)} \right]C{l_2},$$ respecitvely are:
A
Linkage and optical
B
Geometrical and linkage
C
Optical and ionization
D
Linkage and geometrical
Answer :
Geometrical and linkage
238. Indicate the complex ion which shows geometrical isomerism.
A
$${\left[ {Cr{{\left( {{H_2}O} \right)}_4}C{l_2}} \right]^ + }$$
B
$$\left[ {Pt{{\left( {N{H_3}} \right)}_3}Cl} \right]$$
C
$${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
D
$${\left[ {Co{{\left( {CN} \right)}_5}\left( {NC} \right)} \right]^{3 - }}$$
Answer :
$${\left[ {Cr{{\left( {{H_2}O} \right)}_4}C{l_2}} \right]^ + }$$
239. The IUPAC name of $$\left[ {Ni{{\left( {N{H_3}} \right)}_4}} \right]\left[ {NiC{l_4}} \right]$$ is
A
Tetrachloronickel (II) - tetraamminenickel (I)
B
Tetraamminenickel (II) - tetrachloronickel (II)
C
Tetraamminenickel (Il) - tetrachloronickelate (II)
D
Tetrachloronickel (II) - tetrachloronickelate (0)
Answer :
Tetraamminenickel (Il) - tetrachloronickelate (II)
240. The geometry of $$Ni{\left( {CO} \right)_4}\,{\text{and}}\,Ni{\left( {PP{h_3}} \right)_2}C{l_2}\,{\text{are}}$$
A
both square planar
B
tetrahedral and square planar, respectively
C
both tetrahedral
D
square planar and tetrahedral, respectively
Answer :
both tetrahedral
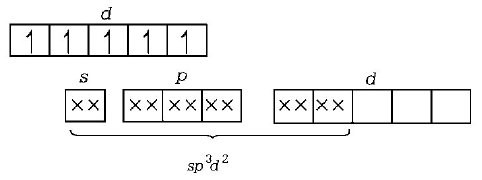
.PNG)
.PNG)
.PNG)