391. Which of the following hydrides is least stable to hydrolysis?
A
$$C{H_4}$$
B
$$Si{H_4}$$
C
$$Sn{H_4}$$
D
$$Pb{H_4}$$
Answer :
$$Si{H_4}$$
392. One mole of calcium phosphide on reaction with excess water gives
A
one mole of phosphine
B
two moles of phosphoric acid
C
two moles of phosphine
D
one mole of phosphorus pentoxide
Answer :
two moles of phosphine
393. Alum helps in purifying water by
A
forming $$Si$$ complex with clay partiles
B
sulphate part which combines with the dirt and removes
it
C
coagulaing the mud particles
D
making mud water soluble.
Answer :
coagulaing the mud particles
394. Which one of the following ionic species has the greatest proton affinity to form stable compound?
A
$$H{S^ - }$$
B
$$NH_2^ - $$
C
$${F^ - }$$
D
$${I^ - }$$
Answer :
$${F^ - }$$
395. Select the correct option regarding the properties of dioxygen.
A
Dioxygen never reacts with metals.
B
Dioxygen is diamagnetic in nature.
C
Combination of dioxygen with other elements is highly exothermic process.
D
Dioxygen liquefies at 55 $$K$$ and freezes at 90 $$K.$$
Answer :
Combination of dioxygen with other elements is highly exothermic process.
396. Which of the following xenon-oxo compounds may not be obtained by hydrolysis of xenon fluorides ?
A
$$Xe{O_2}{F_2}$$
B
$$XeO{F_4}$$
C
$$Xe{O_3}$$
D
$$Xe{O_4}$$
Answer :
$$Xe{O_4}$$
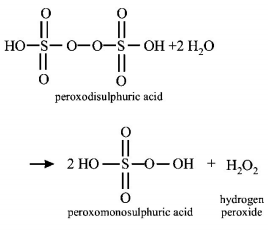
397. Hydrolysis of one mole of peroxodisulphuric acid produces
A
two moles of sulphuric acid
B
two moles of peroxomonosulphuric acid
C
one mole of sulphuric acid and one mole of peroxomonosulphuric acid
D
one mole of sulphuric acid, one mole of
peroxomonosulphuric acid and one mole of hydrogen peroxide.
Answer :
one mole of sulphuric acid, one mole of
peroxomonosulphuric acid and one mole of hydrogen peroxide.
398. The type of hybridisation of boron in diborane is
A
$$sp$$ - hybridisation
B
$$s{p^2}$$ - hybridisation
C
$$s{p^3}$$ - hybridisation
D
$$s{p^3}{d^2}$$ - hybridisation
Answer :
$$s{p^3}$$ - hybridisation
399. Which of the following is not matched correctly with its use?
A
Piezoelectric material - Quartz
B
Ion -exchangers - Graphite
C
Filtration plants - Silica
D
Electrical insulators - Silicones
Answer :
Ion -exchangers - Graphite
400. $$Zn$$ gives $${H_2}$$ gas with $${H_2}S{O_4}$$ and $$HCl$$ but not with $$HN{O_3}$$ because
A
$$Zn$$ act as oxidising agent when react with $$HN{O_3}$$
B
$$HN{O_3}$$ is weaker acid than $${H_2}S{O_4}$$ and $$HCl$$
C
In electrochemical series $$Zn$$ is placed above hydrogen
D
$$NO_3^ - $$ is reduced in preference to hydronium ion
Answer :
$$NO_3^ - $$ is reduced in preference to hydronium ion