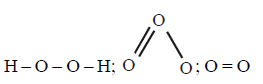411.
Which of the following statements is/are true?
(i) Sulphur exhibits 6 coordination number in its compound.
(ii) Bond energy of $${F_2}$$ is less than $$C{l_2}.$$
(iii) $$PC{l_5}$$ does not exist but $$NC{l_5}$$ exists.
(iv) Elements of 15th group show only +3 and +5 oxidation states.
A
(i) and (ii)
B
(ii) only
C
(ii) and (iii)
D
All of these
Answer :
(i) and (ii)
412. Correct order of $$O - O$$ bond length (increasing) in $${O_2},{H_2}{O_2}$$ and $${O_3}$$ is
A
$${H_2}{O_2} < {O_3} < {O_2}$$
B
$${O_2} < {O_3} < {H_2}{O_2}$$
C
$${O_3} < {O_2} < {H_2}{O_2}$$
D
$${O_3} < {H_2}{O_2} < {O_2}$$
Answer :
$${O_2} < {O_3} < {H_2}{O_2}$$
413. Borax-bead test is responded by
A
divalent metals
B
heavy metals
C
light metals
D
metals which form coloured metaborates
Answer :
metals which form coloured metaborates
414. Mark the example which is not correct.
A
Non-combustible heavy liquid used as fire extinguisher - $$CC{l_4}$$
B
Blocks used to shield radioactive materials - Lead
C
Element which has property of leaving mark on paper - Graphite
D
A gas in solid form used as a refrigerant - Carbon monoxide
Answer :
A gas in solid form used as a refrigerant - Carbon monoxide
415. Acidity of diprotic acids in aqueous solutions increases in the order
A
$${H_2}S < {H_2}Se < {H_2}Te$$
B
$${H_2}Se < {H_2}S < {H_2}Te$$
C
$${H_2}Te < {H_2}S < {H_2}Se$$
D
$${H_2}Se < {H_2}Te < {H_2}S$$
Answer :
$${H_2}S < {H_2}Se < {H_2}Te$$
416. The structural formula of hypophosphorous acid is
A
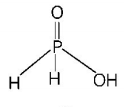

B
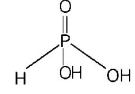

C
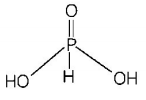

D
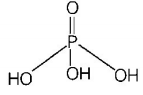

Answer :


417. Which of these is not a monomer for a high molecular mass silicon polymer?
A
$$MeSiC{l_3}$$
B
$$M{e_2}SiC{l_2}$$
C
$$M{e_3}SiCl$$
D
$$PhSiC{l_3}$$
Answer :
$$M{e_3}SiCl$$
418. The acid which has a peroxy linkage is
A
sulphurous acid
B
pyrosulphuric acid
C
dithionic acid
D
Caro’s acid
Answer :
Caro’s acid
419. Which one of the following arrangements does not give the correct picture of the trends indicated against it?
A
$${F_2} > C{l_2} > B{r_2} > {I_2}$$ Oxidising power
B
$${F_2} > C{l_2} > B{r_2} > {I_2}$$ Electron gain enthapy
C
$${F_2} > C{l_2} > B{r_2} > {I_2}$$ Bond dissociation energy
D
$${F_2} > C{l_2} > B{r_2} > {I_2}$$ Electronegativity
Answer :
$${F_2} > C{l_2} > B{r_2} > {I_2}$$ Bond dissociation energy
420. $$C{O_2}$$ and $${N_2}$$ are non-supporters of combustion. However for putting out fires $$C{O_2}$$ is preferred over $${N_2}$$ because $$C{O_2}$$
A
does not burn
B
forms non-combustible products with burning substances
C
is denser than nitrogen
D
is a more reactive gas
Answer :
is denser than nitrogen