421. Which of the following compounds will not give ammonia on heating?
A
$${\left( {N{H_4}} \right)_2}S{O_4}$$
B
$$N{H_2}CON{H_2}$$
C
$$N{H_4}N{O_2}$$
D
$$N{H_4}Cl$$
Answer :
$$N{H_4}N{O_2}$$
422. Which of the following will be formed, if we heat an aqueous solution of $$AlC{l_3}$$ to dryness?
A
$${\text{Solid}}\,AlC{l_3}$$
B
$${\text{Dimer}}\,A{l_2}C{l_6}$$
C
$$Al{\left( {OH} \right)_3}$$
D
$$A{l_2}C{l_3}$$
Answer :
$${\text{Dimer}}\,A{l_2}C{l_6}$$
423. Which of the following is a nitric acid anhydride?
A
$$NO$$
B
$$N{O_2}$$
C
$${N_2}{O_5}$$
D
$${N_2}{O_3}$$
Answer :
$${N_2}{O_5}$$
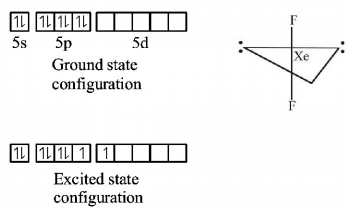
424. Which statement about noble gases is not correct ?
A
$$Xe$$ forms $$Xe{F_6}.$$
B
$$Ar$$ is used in electric bulbs.
C
$$Kr$$ is obtained during radioactive disintegration.
D
$$He$$ has the lowest $$b.p.$$ among all the noble gases.
Answer :
$$Kr$$ is obtained during radioactive disintegration.
425. Which of the following statements is false ?
A
Water gas is a mixture of hydrogen and carbon monoxide
B
Producer gas is a mixture of $$CO$$ and nitrogen
C
Water gas is a mixture of water vapour and hydrogen
D
Natural gas consists of methane, ethane and gaseous hydrocarbons.
Answer :
Water gas is a mixture of hydrogen and carbon monoxide
426. Which one of the following is not the characteristic property of carbon?
A
It exhibits catenation.
B
It forms compounds with multiple bonds.
C
Its melting point and boiling point are exceptionally high.
D
It shows semi-metallic character.
Answer :
It shows semi-metallic character.
427. Oxidation of thiosulphate by iodine gives
A
tetrathionate ion
B
sulphide ion
C
sulphate ion
D
sulphite ion
Answer :
tetrathionate ion
428.
What are $$X$$ and $$Y$$ in the reaction?
$$3{B_2}{H_6} + 6X \to 3{\left[ {B{H_2}{{\left( X \right)}_2}} \right]^ + }$$ \[{{\left[ B{{H}_{4}} \right]}^{-}}\xrightarrow{\text{heat}}Y+12{{H}_{2}}\]
A
$$X = N{H_3},Y = {B_3}{N_3}{H_6}$$
B
$$X = CO,Y = B{H_3}CO$$
C
$$X = NaH,Y = NaF$$
D
$$X = N{F_3},Y = {B_3}{N_3}$$
Answer :
$$X = N{H_3},Y = {B_3}{N_3}{H_6}$$
429. In $$Xe{F_2},\,Xe{F_4},Xe{F_6}$$ the number of lone pairs on $$Xe$$ are respectively
A
$$2,3,1$$
B
$$1,2,3$$
C
$$4,1,2$$
D
$$3,2,1$$
Answer :
$$3,2,1$$
430. $$Xe{F_6}$$ dissolves in anhydrous $$HF$$ to give a good conducting solution which contains :
A
$${H^ + }\,{\text{and}}\,\,XeF_7^ - \,ion$$
B
$$HF_2^ - \,{\text{and}}\,\,XeF_5^ + \,ions$$
C
$$HXeF_6^ + \,{\text{and}}\,\,{F^ - }\,ions$$
D
$${\text{none of these}}$$
Answer :
$$HF_2^ - \,{\text{and}}\,\,XeF_5^ + \,ions$$
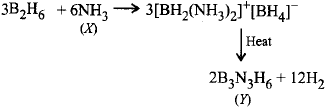
.PNG)
.PNG)