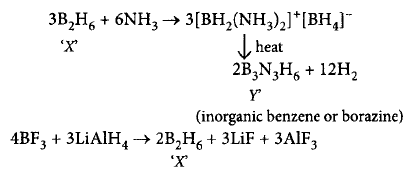451. Among the following compounds, which on heating do not produce $${N_2}?$$
A
$${\left( {N{H_4}} \right)_2}C{r_2}{O_7}$$
B
$$N{H_4}Cl + NaN{O_2}$$
C
$$N{H_4}Cl + CaO$$
D
$$Ba{\left( {{N_3}} \right)_2}$$
Answer :
$$N{H_4}Cl + CaO$$
452. Which is not the use of orthoboric acid?
A
As an antiseptic and eye wash.
B
In glass industry.
C
In glazes for pottery.
D
In borax - bead test.
Answer :
In borax - bead test.
453. A compound $$X,$$ of boron reacts with $$N{H_3}$$ on heating to give another compound $$Y$$ which is called inorganic benzene. The compound $$X$$ can be prepared by treating $$B{F_3}$$ with lithium aluminium hydride. The compounds $$X$$ and $$Y$$ are represented by the formulas
A
$${B_2}{H_6},{B_3}{N_3}{H_6}$$
B
$${B_2}{O_3},{B_3}{N_3}{H_6}$$
C
$$B{F_3},{B_3}{N_3}{H_6}$$
D
$${B_3}{N_3}{H_6},{B_2}{H_6}$$
Answer :
$${B_2}{H_6},{B_3}{N_3}{H_6}$$
454. When $$Al$$ is added to $$NaOH$$ solution
A
No action takes place
B
$$NaAl{O_2}$$ is formed and $${H_2}$$ is evolved
C
$$Al{\left( {OH} \right)_3}$$ is formed and $${H_2}$$ is evolved
D
$$N{a_2}Al{O_2}$$ is formed and $${H_2}$$ is evolved
Answer :
$$NaAl{O_2}$$ is formed and $${H_2}$$ is evolved
455. Carbon monoxide acts as a donor and reacts with certain metals to give metal carbonyls. This is due to
A
presence of one sigma and two $$pi$$ bonds between $$C$$ and $$O\left( {:C \equiv O:} \right)$$
B
presence of a lone pair on carbon atom in $$CO$$ molecule
C
presence of lone pair on oxygen atom in $$CO$$ molecule
D
poisonous nature of $$CO$$
Answer :
presence of a lone pair on carbon atom in $$CO$$ molecule
456.
\[SiC{{l}_{4}}\xrightarrow{{{H}_{2}}O}X\xrightarrow{\text{Heat}}Y\] \[\xrightarrow{NaOH}Z\]
$$X, Y$$ and $$Z$$ in the above reaction are
| X | Y | Z | |
|---|---|---|---|
| (a) | $$Si{O_2}$$ | $$Si$$ | $$NaSi$$ |
| (b) | $$Si{\left( {OH} \right)_4}$$ | $$Si{O_2}$$ | $$N{a_2}Si{O_3}$$ |
| (c) | $$Si{\left( {OH} \right)_4}$$ | $$Si$$ | $$Si{O_2}$$ | (d) | $$Si{O_2}$$ | $$SiC{l_4}$$ | $$N{a_2}Si{O_3}$$ |
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(b)
457. Water gas is produced by
A
passing steam through a red hot coke bed
B
saturating hydrogen with moisture
C
mixing oxygen and hydrogen in the ratio of 1 : 2
D
heating a mixture of $$C{O_2}\,$$ and $$C{H_4}$$ in petroleum refineries
Answer :
passing steam through a red hot coke bed
458. Oxidation states of $$P$$ in $${H_4}{P_2}{O_5},{H_4}{P_2}{O_6}$$ and $$\,{H_4}{P_2}{O_7},$$ respectively are
A
$$+3,+ 5$$ and $$+ 4$$
B
$$+5,+ 3$$ and $$+4$$
C
$$+5, + 4$$ and $$+3$$
D
$$+3, + 4$$ and $$+5$$
Answer :
$$+3, + 4$$ and $$+5$$
459. Silicon has a strong tendency to form polymers like silicones. The chain length of silicone polymer can be controlled by adding
A
$$MeSiC{l_3}$$
B
$$M{e_2}SiC{l_2}$$
C
$$M{e_3}SiCl$$
D
$$M{e_4}Si$$
Answer :
$$M{e_3}SiCl$$
460. The deep blue colour produced on adding excess of ammonia to copper sulphate is due to presence of
A
$$C{u^{2 + }}$$
B
$${\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}$$
C
$${\left[ {Cu{{\left( {N{H_3}} \right)}_6}} \right]^{2 + }}$$
D
$${\left[ {Cu{{\left( {N{H_3}} \right)}_2}} \right]^{2 + }}$$
Answer :
$${\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}$$