511. Which is the correct arrangement of the compounds based on their bond strength?
A
$$HF > HCl > HBr > HI$$
B
$$HI > HBr > HCl > HF$$
C
$$HCl > HF > HBr > HI$$
D
$$HF > HBr > HCl > HI$$
Answer :
$$HF > HCl > HBr > HI$$
512.
Fill in the blanks.
The high reactivity of fluorine is due to its ________ dissociation energy. Its shows only ________ oxidation state. It has ________ electron affinity than chlorine. Among all hydrogen halides boiling point is highest for ________ .
A
low, - 1, lower, $$HF$$
B
high, + 1, higher, $$HF$$
C
low, + 1, lower, $$HCl$$
D
high, - 1, higher, $$HF$$
Answer :
low, - 1, lower, $$HF$$
513. Anhydrous aluminium chloride $$\left( {A{l_2}C{l_6}} \right)$$ is covalent compound and soluble in water giving :
A
$$A{l^{3 + }}\,{\text{and}}\,C{l^ - }\,ions$$
B
$${\left[ {Al{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}\,{\text{and}}\,C{l^ - }\,ions$$
C
$${\left[ {AlC{l_2}{{\left( {{H_2}O} \right)}_4}} \right]^ + }{\text{and}}{\left[ {AlC{l_4}{{\left( {{H_2}O} \right)}_2}} \right]^ - }ions$$
D
none of the above
Answer :
$${\left[ {AlC{l_2}{{\left( {{H_2}O} \right)}_4}} \right]^ + }{\text{and}}{\left[ {AlC{l_4}{{\left( {{H_2}O} \right)}_2}} \right]^ - }ions$$
514. In graphite, the layers of carbon atoms are held by
A
covalent bonds
B
coordinate bonds
C
van der Waals' forces
D
ionic bonds
Answer :
van der Waals' forces
515.
Which of the following is the best description for the behaviour of bromine in the reaction given below ?
$${H_2}O + B{r_2} \to HOBr + HBr$$
A
Proton acceptor only
B
Both oxidized and reduced
C
Oxidized only
D
Reduced only
Answer :
Both oxidized and reduced
516.
In the following sets of reactants which two sets best exhibit the amphoteric characters of $$A{l_2}{O_3}.\,x{H_2}O?$$
$$\eqalign{
& {\text{Set}}\,1{\text{:}}\,A{l_2}{O_3}.\,x{H_2}O\left( s \right)\,{\text{and}}\,O{H^ - }\left( {aq} \right) \cr
& {\text{Set}}\,{\text{2:}}\,A{l_2}{O_3}.\,x{H_2}O\left( s \right){\text{and}}\,{{\text{H}}_2}O\left( l \right) \cr
& {\text{Set}}\,{\text{3:}}\,A{l_2}{O_3}.\,x{H_2}O\left( s \right)\,{\text{and}}\,{H^ + }\left( {aq} \right) \cr
& {\text{Set}}\,{\text{4:}}\,A{l_2}{O_3}.\,x{H_2}O\left( s \right)\,{\text{and}}\,N{H_3}\left( {aq} \right) \cr} $$
A
1 and 2
B
1 and 3
C
2 and 4
D
3 and 4
Answer :
1 and 3
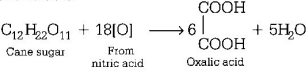
517. Cane sugar on reaction with nitric acid gives
A
$$C{O_2}\,\,{\text{and}}\,\,S{O_2}$$
B
$$2HCOOH$$
C
$${\left( {COOH} \right)_2}$$
D
$${\text{no reaction}}$$
Answer :
$${\left( {COOH} \right)_2}$$
518. $${P_2}{H_4}$$ can be removed from phosphine containing traces of it
A
by passing impure $$P{H_3}$$ gas through a freezing mixture.
B
by passing the impure $$P{H_3}$$ gas through $$HI$$ and then its treatment with $$KOH\left( {aq} \right).$$
C
by both (A) and (B).
D
by none of these.
Answer :
by both (A) and (B).
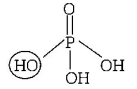
519. Basicity of orthophosphoric acid is
A
2
B
3
C
4
D
5
Answer :
3
520. Identify the incorrect statement.
A
Graphite is thermodynamically most stable allotrope of carbon.
B
Other forms of elemental carbon like coke, carbon black, charcoal are impure forms of graphite.
C
All allotropes of carbon have thermodynamically different stability.
D
Charcoal and coke are obtained by heating wood in absence of air.
Answer :
Charcoal and coke are obtained by heating wood in absence of air.