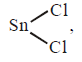601. Which of the following oxidation states are the most characteristics for lead and tin respectively?
A
+ 4, + 2
B
+ 2, + 4
C
+ 4, + 4
D
+ 2, + 2
Answer :
+ 2, + 4
602. The shape of gaseous $$SnC{l_2}$$ is
A
tetrahedral
B
linear
C
angular
D
$$T$$ - shaped
Answer :
angular
603. The correct order of acidic strength is
A
$${K_2}O > CaO > MgO$$
B
$$C{O_2} > {N_2}{O_5} > S{O_3}$$
C
$$N{a_2}O > MgO > A{l_2}{O_3}$$
D
$$C{l_2}{O_7} > S{O_2} > {P_4}{O_{10}}$$
Answer :
$$C{l_2}{O_7} > S{O_2} > {P_4}{O_{10}}$$
604. Which of the following is not correctly matched?
A
$$PC{l_5} - s{p^3}d\,{\text{hybridisation}}$$
B
$$PC{l_3} - s{p^3}\,{\text{hybridisation}}$$
C
$$PC{l_5}\left( {{\text{solid}}} \right) - {\left[ {PC{l_4}} \right]^ + }{\left[ {PC{l_6}} \right]^ - }$$
D
$$PC{l_5} - {\text{brownish powder}}$$
Answer :
$$PC{l_5} - {\text{brownish powder}}$$
605. In the preparation of $$HN{O_3},$$ we get $$NO$$ gas by catalytic oxidation of ammonia. The moles of $$NO$$ produced by the oxidation of two moles of $$N{H_3}$$ will be ________.
A
2
B
3
C
4
D
6
Answer :
2
606. The gas evolved on heating $$Ca{F_2}$$ and $$Si{O_2}$$ with concentrated $${H_2}S{O_4},$$ on hydrolysis gives a white gelatinous precipitate. The precipitate is :
A
hydrofluosilicic acid
B
silica gel
C
silicic acid
D
calciumfluorosilicate
Answer :
calciumfluorosilicate
607. Which among the following factors is the most important in making fluorine the strongest oxidizing halogen?
A
Hydration enthalpy
B
Ionization enthalpy
C
Electron affinity
D
Bond dissociation energy
Answer :
Bond dissociation energy
608. The formation of $$O_2^ + {\left[ {Pt{F_6}} \right]^ - }$$ is the basis for the formation of xenon fluorides. This is because
A
$${O_2}$$ and $$Xe$$ have comparable sizes.
B
both $${O_2}$$ and $$Xe$$ are gases.
C
$${O_2}$$ and $$Xe$$ have comparable ionisation energies.
D
Both (A) and (C).
Answer :
Both (A) and (C).
609. The substance used as a smoke screen in warfare is
A
$$SiC{l_4}$$
B
$$P{H_3}$$
C
$$PC{l_5}$$
D
$${\text{acetylene}}$$
Answer :
$$SiC{l_4}$$
610. Which of the following is not correctly matched ?
A
$$S{F_4} - {\text{gas}}$$
B
$$Se{F_4} - {\text{liquid}}$$
C
$$Te{F_4} - {\text{solid}}$$
D
$$S{F_6} - {\text{solid}}$$
Answer :
$$S{F_6} - {\text{solid}}$$