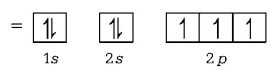
611. Which of the following is used as protective shields in nuclear industries?
A
$$^{27}Al$$
B
$$^{10}B$$
C
$$^{16}O$$
D
$$^{14}C$$
Answer :
$$^{10}B$$
612. Which of the following is a Lewis acid?
A
$$AlC{l_3}$$
B
$$MgC{l_2}$$
C
$$CaC{l_2}$$
D
$$BaC{l_2}$$
Answer :
$$AlC{l_3}$$
613. Aqueous solution of ammonia consists of
A
$${H^ + }$$
B
$$O{H^ - }$$
C
$$NH_4^ + $$
D
$$NH_4^ + \,{\text{and}}\,O{H^ - }$$
Answer :
$$NH_4^ + \,{\text{and}}\,O{H^ - }$$
614.
\[\begin{align}
& M{{g}_{3}}{{B}_{2}}\xrightarrow{HC{{l}_{\left( aq \right)}}}\left[ X \right]+MgC{{l}_{2}} \\
& \left[ X \right]+{{H}_{2}}O\xrightarrow{HC{{l}_{\left( aq \right)}}}\left[ Y \right]+{{H}_{2}} \\
\end{align}\]
For $$\left[ X \right]$$ and $$\left[ Y \right]$$ the incorrect choice is
A
$$\left[ X \right]$$ is $${B_2}{H_6}$$ and $$\left[ Y \right]$$ is $${H_3}B{O_3}$$
B
$$\left[ X \right]$$ with air and $$\left[ Y \right]$$ on strong heating ( red heat ) give same compound
C
In $$\left[ Y \right],$$ boron completes its octet by removing $${H^ + }$$ from water molecule
D
None of these
Answer :
None of these
615.
Complete the following reactions by filling the appropriate choice.
$$\left( {\text{A}} \right)6Xe{F_4} + 12{H_2}O \to $$ $$4Xe + 2Xe{O_3} + \underline {\left( {\text{i}} \right)} + \underline {\left( {{\text{ii}}} \right)} $$
$$\left( {\text{B}} \right)Xe{F_6} + 3{H_2}O \to \underline {\left( {{\text{iii}}} \right)} + 6HF$$
| (i) | (ii) | (iii) | |
|---|---|---|---|
| (a) | $${F_2}$$ | $${H_2}O$$ | $$XeO{F_4}$$ |
| (b) | $$24HF$$ | $$3{O_2}$$ | $$Xe{O_3}$$ |
| (c) | $$2HF$$ | $$2{H_2}O$$ | $$XeO$$ |
| (d) | $$HF$$ | $${H_2}O$$ | $$X{e_2}{O_3}$$ |
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(b)
616. Which of the following statements is not correct for nitrogen?
A
Its electronegativity is very high
B
$$d$$-orbitals are available for bonding
C
It is a typical non-metal
D
Its molecular size is small
Answer :
$$d$$-orbitals are available for bonding
617. Bleaching powder reacts with a few drops of $$conc.$$ $$HCl$$ to give
A
chlorine
B
hypochlorous acid
C
calcium oxide
D
oxygen
Answer :
chlorine
618. A solution of $$KBr$$ is treated with each of the following. Which one would liberate bromine
A
$$C{l_2}$$
B
$$HI\,$$
C
$${I_2}$$
D
$$S{O_2}$$
Answer :
$$C{l_2}$$
619.
Bond angle in $${H_2}O\left( {{{104.5}^ \circ }} \right)$$ is higher than the bond angle of $${H_2}S\left( {{{92.1}^ \circ }} \right).$$ The difference is due to

A
$$O$$ is diatomic and $$S$$ is tetra-atomic
B
difference in electronegativity of $$S$$ and $$O$$
C
difference in oxidation states of $$S$$ and $$O$$
D
difference in shapes of hybrid orbitals of $$S$$ and $$O.$$
Answer :
difference in electronegativity of $$S$$ and $$O$$
620. Which of the following oxides is anhydride of nitrous acid?
A
$${N_2}{O_3}$$
B
$$N{O_2}$$
C
$$NO$$
D
$${N_2}{O_4}$$
Answer :
$${N_2}{O_3}$$