631.
$$Al{F_3}$$ is soluble in $$HF$$ only in presence of $$KF.$$
It is due to the formation of
A
$${K_3}\left[ {Al{F_3}{H_3}} \right]$$
B
$${K_3}\left[ {Al{F_6}} \right]$$
C
$$Al{H_3}$$
D
$$K\left[ {Al{F_3}H} \right]$$
Answer :
$${K_3}\left[ {Al{F_6}} \right]$$
632. In borax bead test which compound is formed?
A
$$Ortho$$ borate
B
$$Meta$$ borate
C
Double oxide
D
Tetra borate
Answer :
$$Meta$$ borate
633. Which of the following phosphorus is the most reactive?
A
Red phosphorus
B
White phosphorus
C
Scarlet phosphorus
D
Violet phosphorus
Answer :
White phosphorus
634. Each of the following is true for white and red phosphorus except that they
A
both are soluble in $$C{S_2}$$
B
can be oxidised by heating in air
C
consist of the same kind of atoms
D
can be converted into one another
Answer :
both are soluble in $$C{S_2}$$
635. Which would quickly absorb oxygen?
A
Alkaline solution of pyrogallol
B
Conc. $${H_2}S{O_4}$$
C
Lime water
D
Alkaline solution of $$CuS{O_4}$$
Answer :
Alkaline solution of pyrogallol
636. In group 13, electronegativity first decreases from $$B$$ to $$Al$$ and then increases marginally down the group. This is because of
A
non-metallic nature of $$B$$
B
discrepancies in atomic size of elements
C
ability of $$B$$ and $$Al$$ to form $$p\pi - p\pi $$ multiple bonds
D
irregular trend in electronegativity throughout the periodic table.
Answer :
discrepancies in atomic size of elements
637. The decreasing order of boiling points of the following hydrides is
A
$$Sb{H_3} > As{H_3} > P{H_3} > N{H_3}$$
B
$$N{H_3} > Sb{H_3} > As{H_3} > P{H_3}$$
C
$$Sb{H_3} > N{H_3} > As{H_3} > P{H_3}$$
D
$$P{H_3} > As{H_3} > Sb{H_3} > N{H_3}$$
Answer :
$$Sb{H_3} > N{H_3} > As{H_3} > P{H_3}$$
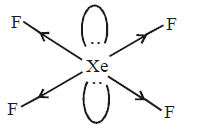
638. The compound of xenon with zero dipole moment is
A
$$Xe{O_3}$$
B
$$Xe{F_4}$$
C
$$XeO{F_4}$$
D
$$Xe{O_2}$$
Answer :
$$Xe{F_4}$$
639. Often a ground glass stopper gets stuck in the neck of a glass bottle containing $$NaOH$$ solution. This is due to :
A
The presence of dirt particles in between.
B
The formation of solid silicate in between by the reaction of $$Si{O_2}$$ of glass with $$NaOH.$$
C
The formation of $$N{a_2}C{O_3}$$ in between by the reaction of $$C{O_2}$$ of air and $$NaOH.$$
D
Glass contains a boron compound which forms a precipitate with the $$NaOH$$ solution.
Answer :
The formation of solid silicate in between by the reaction of $$Si{O_2}$$ of glass with $$NaOH.$$
640. Chlorine water on standing loses its colour and forms :
A
$$HCl\,\,{\text{only}}$$
B
$$HCl\,\,{\text{and}}\,\,HCl{O_2}$$
C
$$HCl\,\,{\text{and}}\,\,HOCl$$
D
$$HOCl\,\,{\text{and}}\,\,HOC{l_2}$$
Answer :
$$HCl\,\,{\text{and}}\,\,HOCl$$