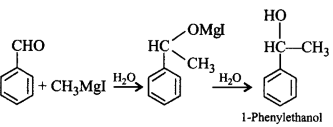
101. 1-Phenylethanol can be prepared by the reaction of benzaldehyde with
A
methyl bromide
B
ethyl iodide and magnesium
C
methyl iodide and magnesium
D
methyl bromide and aluminium bromide
Answer :
methyl iodide and magnesium
102.
In the reaction :
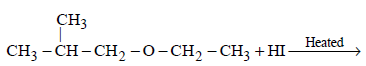
Which of the following compounds will be formed ?
A
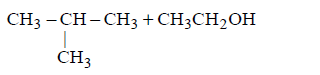
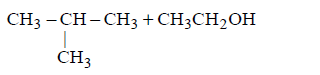
B


C
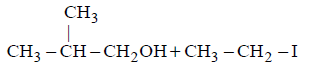
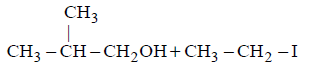
D


Answer :
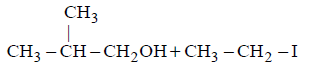
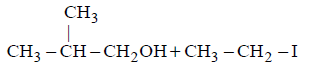
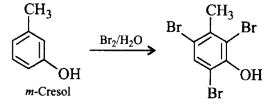
103. Which of the following compounds will give tribromo derivative on treatment with bromine water?
A


B


C


D
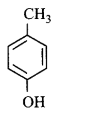

Answer :


104. Ethyl chloride is converted into diethyl ether by
A
Wurtz synthesis
B
Grignard reaction
C
Perkin’s reaction
D
Williamson's synthesis
Answer :
Williamson's synthesis
105. Phenol reacts with bromine in carbon disulphide at low temperature to give
A
$$m$$ - bromophenol
B
$$o$$ - and $$p$$ - bromophenol
C
$$p$$ - bromophenol
D
2, 4, 6 - tribromophenol
Answer :
$$o$$ - and $$p$$ - bromophenol
106.
Which of the following compounds is aromatic alcohol?
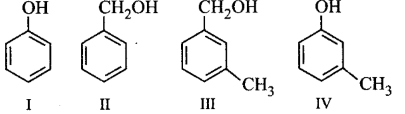
A
I, II, III, IV
B
I, IV
C
II, III
D
I
Answer :
II, III
107.
Dehydration of the following in increasing order is


A
I < II < III < IV
B
II < III < IV < I
C
I < III < IV < II
D
None of these
Answer :
I < II < III < IV
108.
Give IUPAC name of the compound given below :
\[C{{H}_{3}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
Cl\,\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}-C{{H}_{2}}\] \[\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OH\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{3}}\]
A
2-Chloro-5-hydroxyhexane
B
2-Hydroxy-5-chlorohexane
C
5-Chlorohexan-2-ol
D
2-Chlorohexan-5-ol
Answer :
5-Chlorohexan-2-ol
109. The boiling point of $$p$$ - nitrophenol is higher than that of $$o$$ - nitrophenol because
A
$$N{O_2}$$ group at $$p$$ - position behaves in a different
way from that at $$o$$ - position
B
intramolecular hydrogen bonding exists in $$p$$ - nitrophenol
C
there is intermolecular hydrogen bonding in $$p$$ - nitrophenol
D
$$p$$ - nitrophenol has a higher molecular weight than $$o$$ - nitrophenol
Answer :
there is intermolecular hydrogen bonding in $$p$$ - nitrophenol
110. Methanol is industrially prepared by
A
oxidation of $$C{H_4}$$ by steam at $${900^ \circ }C$$
B
reduction of $$HCHO$$ using $$LiAl{H_4}$$
C
reaction of $$HCHO$$ with a solution of $$NaOH$$
D
reduction of $$CO$$ using $${H_2}$$ and $$ZnO - C{r_2}{O_3}$$
Answer :
reduction of $$CO$$ using $${H_2}$$ and $$ZnO - C{r_2}{O_3}$$



.PNG)
