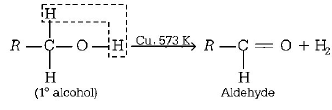
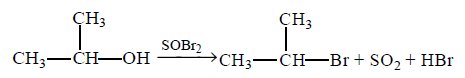131. What is formed when a primary alcohol undergoes catalytic dehydrogenation?
A
Aldehyde
B
Ketone
C
Alkene
D
Acid
Answer :
Aldehyde
132. $$3\,moles$$ of ethanol react with one mole of phosphorus tribromide to form $$3\,moles$$ of bromoethane and one mole of $$X.$$ Which of the following is $$X?$$
A
$${H_3}P{O_4}$$
B
$${H_3}P{O_2}$$
C
$$HP{O_3}$$
D
$${H_3}P{O_3}$$
Answer :
$${H_3}P{O_3}$$
133. In allylic and benzylic alcohols, $$-OH$$ group is attached to
A
$$s{p^3},s{p^3}$$ - hybridised carbon atom respectively
B
$$s{p^2},s{p^3}$$ - hybridised carbon atom respectively
C
$$sp,s{p^2}$$ - hybridised carbon atom respectively
D
$$s{p^2},sp$$ - hybridised carbon atom respectively
Answer :
$$s{p^3},s{p^3}$$ - hybridised carbon atom respectively
134. The compound which reacts fastest with Lucas reagent is ( at room temperature )
A
butan-1-$$ol$$
B
butan-2-$$ol$$
C
2-methyl propan-1-$$ol$$
D
2-methyl propan-2-$$ol$$
Answer :
2-methyl propan-2-$$ol$$
135. Ethers have lower boiling points than their corresponding isomeric alcohols because of
A
hydrogen bonding in alcohols that is absent in ethers due to low polarity
B
hydrogen bonding in ethers due to high polarity
C
insolubility of ethers in water due to less polarity
D
inertness of ethers as compared to alcohols
Answer :
hydrogen bonding in alcohols that is absent in ethers due to low polarity
136. Methyl alcohol is industrially prepared by the action of
A
$$C{H_3}COC{H_3}$$
B
$$CO + {H_2}$$
C
$$C{H_3}COOH$$
D
$${C_2}{H_5}OH$$
Answer :
$$CO + {H_2}$$
137. A compound $$X$$ with the molecular formula, $${C_3}{H_8}O$$ can be oxidised to another compound $$Y$$ whose molecular formula is $${C_3}{H_6}{O_2}.$$ The compound $$X$$ may be
A
$$C{H_3}C{H_2}OC{H_3}$$
B
$$C{H_3}C{H_2}CHO$$
C
$$C{H_3}C{H_2}C{H_2}OH$$
D
$$C{H_3}CHOHC{H_3}$$
Answer :
$$C{H_3}C{H_2}C{H_2}OH$$
138. Phenol when treated with excess of bromine water gives a white precipitate of
A
2, 4, 6 - tribromophenol
B
$$o$$ - bromophenol
C
$$p$$ - bromophenol
D
bromobenzene
Answer :
2, 4, 6 - tribromophenol
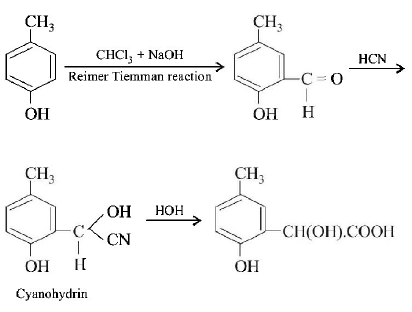
139. p - cresol reacts with chloroform in alkaline medium to give the compound $$A$$ which adds hydrogen cyanide to form, the compound $$B.$$ The latter on acidic hydrolysis gives chiral carboxylic acid. The structure of the carboxylic acid is
A
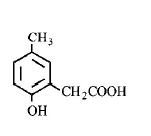

B
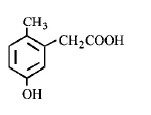

C


D
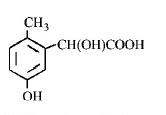

Answer :


140.
Which is the best reagent to convert isopropyl alcohol to isopropyl bromide ?
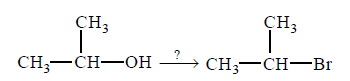
A
$$HBr$$
B
$$SOB{r_2}$$
C
$$B{r_2}$$
D
$$C{H_3}MgBr$$
Answer :
$$SOB{r_2}$$