151. Diethyl ether on heating with conc. $$HI$$ gives two moles of $$H$$
A
ethanol
B
iodoform
C
ethyl iodide
D
methyl iodide
Answer :
ethyl iodide
152.
The product of the following reaction, 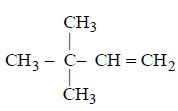 \[\xrightarrow[\left( \text{ii} \right)\,NaB{{H}_{4}}]{\left( \text{i} \right)\,Hg{{\left( OAc \right)}_{2}},{{H}_{2}}O}\] is
\[\xrightarrow[\left( \text{ii} \right)\,NaB{{H}_{4}}]{\left( \text{i} \right)\,Hg{{\left( OAc \right)}_{2}},{{H}_{2}}O}\] is
A
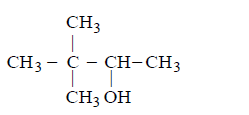
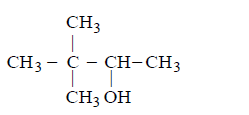
B


C


D
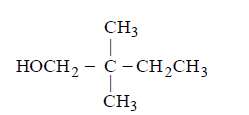
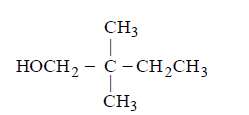
Answer :
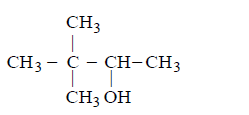
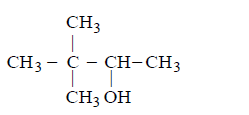
153.
The acidic hydrolysis of ether $$(X)$$ shown below is fastest
when
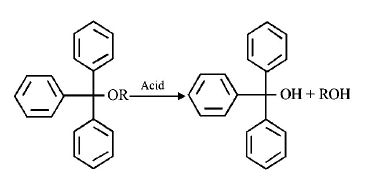
A
One phenyl group is replaced by a methyl group
B
One phenyl group is replaced by a para - methoxyphenyl
group
C
Two phenyl groups are replaced by two
para-methoxyphenyl groups
D
No structural change is made to $$X$$
Answer :
Two phenyl groups are replaced by two
para-methoxyphenyl groups
154.
Identify $$Z$$ in the sequence :
\[C{{H}_{3}}C{{H}_{2}}CH=C{{H}_{2}}\xrightarrow{HBr/{{H}_{2}}{{O}_{2}}}Y\xrightarrow{{{C}_{2}}{{H}_{5}}ONa}Z\]
A
$${\left( {C{H_3}} \right)_2}COHC{H_2}C{H_3}$$
B
$$C{H_3}C{H_2}CH\left( {C{H_3}} \right) - O - C{H_2}C{H_3}$$
C
$$C{H_3}{\left( {C{H_2}} \right)_3} - O - C{H_2}C{H_3}$$
D
$$C{H_3}{\left( {C{H_2}} \right)_4} - O - C{H_3}$$
Answer :
$$C{H_3}{\left( {C{H_2}} \right)_3} - O - C{H_2}C{H_3}$$
155.
The structure of the product formed in the reaction given below is :

A
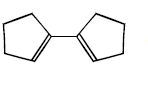

B


C
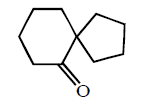

D


Answer :


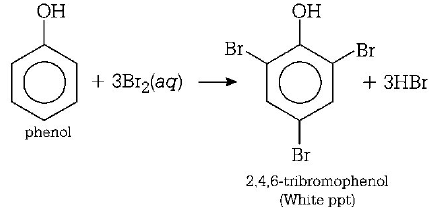
156. When phenol is treated with excess of bromine water, it gives
A
$$m$$ - bromophenol
B
$$o$$ - and $$p$$ - bromophenols
C
2, 4 - dibromophenol
D
2, 4, 6 - tribromophenol
Answer :
2, 4, 6 - tribromophenol
157. The ether that undergoes electrophilic substitution reaction is
A
$$C{H_3}O{C_2}{H_5}$$
B
$${C_6}{H_5}OC{H_3}$$
C
$$C{H_3}OC{H_3}$$
D
$${C_2}{H_5}O{C_2}{H_5}$$
Answer :
$${C_6}{H_5}OC{H_3}$$
158. Which one is the most acidic compound?
A


B
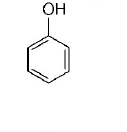

C
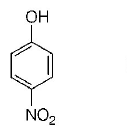

D
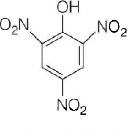

Answer :


159.
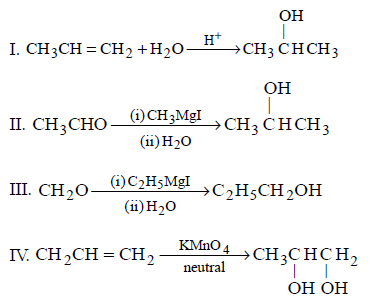
Which one/ones of the following reactions will yield 2-propanol ?
\[\begin{align}
& \left( \text{I} \right)C{{H}_{2}}=CH-C{{H}_{3}}+{{H}_{2}}O\xrightarrow{{{H}^{+}}} \\
& \left( \text{II} \right)C{{H}_{3}}-CHO\xrightarrow[\left( \text{ii} \right)\,{{H}_{2}}O]{\left( \text{i} \right)\,C{{H}_{3}}MgI} \\
& \left( \text{III} \right)C{{H}_{2}}O\xrightarrow[\left( \text{ii} \right)\,{{H}_{2}}O]{\left( \text{i} \right)\,{{C}_{2}}{{H}_{5}}MgI} \\
& \left( \text{IV} \right)C{{H}_{2}}=CH-C{{H}_{3}}\xrightarrow{\text{Neutral}\,KMn{{O}_{4}}} \\
\end{align}\]
A
I and II
B
II and III
C
III and I
D
II and IV
Answer :
I and II
160. An unknown alcohol is treated with the “Lucas reagent” to determine whether the alcohol is primary, secondary or tertiary. Which alcohol reacts fastest and by what mechanism :
A
secondary alcohol by $${S_N}1$$
B
tertiary alcohol by $${S_N}1$$
C
secondary alcohol by $${S_N}2$$
D
tertiary alcohol by $${S_N}2$$
Answer :
tertiary alcohol by $${S_N}1$$