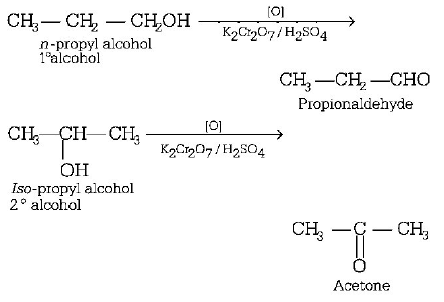
201. $$n$$ - propyl alcohol and $$iso$$ - propyl alcohol can be chemically distinguished by which reagent?
A
$$PC{l_5}$$
B
reduction
C
oxidation with potassium dichromate
D
ozonolysis
Answer :
oxidation with potassium dichromate
202.
Arrange the following compounds in increasing order of boiling point.
Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol
A
Propan-1-ol, butan-2-ol, butan-1-ol, pentan -1-ol
B
Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol
C
Pentan-1-ol, butan-2-ol, butan-1-ol, propan-1-ol
D
Pentan-1-ol, butan-1-ol, butan-2-ol, propan-1-ol
Answer :
Propan-1-ol, butan-2-ol, butan-1-ol, pentan -1-ol
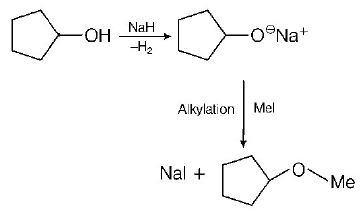
203.
The reaction

can be classified as
A
Alcohol formation reaction
B
Dehydration reaction
C
Williamson alcohol synthesis reaction
D
Williamson ether synthesis reaction
Answer :
Williamson ether synthesis reaction
204. The best method to prepare cyclohexene from cyclohexanol is by using
A
$$Conc.\,HCl + ZnC{l_2}$$
B
$$Conc.\,{H_3}P{O_4}$$
C
$$HBr$$
D
$$Conc.\,HCl$$
Answer :
$$Conc.\,{H_3}P{O_4}$$
205. An organic compound $$A$$ containing $$C, H$$ and $$O$$ has a pleasant odour with boiling point of $${78^ \circ }C.$$ On boiling $$A$$ with concentrated $${H_2}S{O_4},$$ a colourless gas is produced which decolourises bromine water and alkaline $$KMn{O_4}.$$ The organic liquid $$A$$ is
A
$${C_2}{H_5}Cl$$
B
$${C_2}{H_5}COOC{H_3}$$
C
$${C_2}{H_5}OH$$
D
$${C_2}{H_6}$$
Answer :
$${C_2}{H_5}OH$$
206.
The increasing order of boiling points of the below
mentioned alcohols is
(I) 1,2 - dihydroxybenzene
(II) 1, 3 - dihydroxybenzene
(II) 1, 4 - dihydroxybenzene
(IV) Hydroxybenzene
A
I < II < IV < III
B
I < II < III < IV
C
IV < II < I < III
D
IV < I < II < III
Answer :
IV < I < II < III
207. Ethyl alcohol is heated with conc $${H_2}S{O_4}$$ the product formed is
A
\[{{H}_{3}}C\underset{\begin{smallmatrix}
\parallel \\
O
\end{smallmatrix}}{\mathop{-C-}}\,O{{C}_{2}}{{H}_{5}}\]
B
$${C_2}{H_6}$$
C
$${C_2}{H_4}$$
D
$${C_2}{H_2}$$
Answer :
$${C_2}{H_4}$$
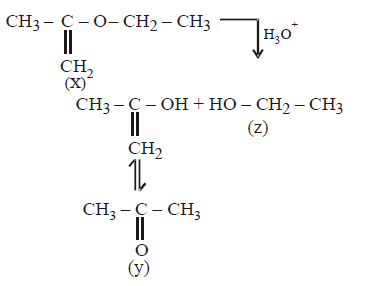
208. \[\underset{\left( {{C}_{5}}{{H}_{10}}O \right)}{\mathop{\left( X \right)}}\,\xrightarrow{{{H}_{3}}{{O}^{+}}}Y+Z\] ( $$Y$$ and $$Z$$ both give \[\left( {{C}_{6}}{{H}_{10}}O \right)\] the Iodoform test). The compound $$X$$ is -
A
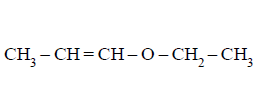
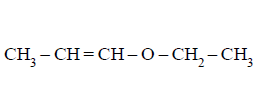
B


C


D


Answer :


209. In which of the following reactions hydrogen gas will not be evolved ?
A


B
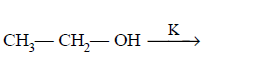
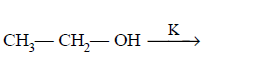
C


D


Answer :


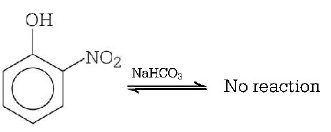
210. Which of the following will not be soluble in sodium hydrogen carbonate?
A
2, 4, 6 - trinitrophenol
B
Benzoic acid
C
$$o$$ - nitrophenol
D
Benzenesulphonic acid
Answer :
$$o$$ - nitrophenol