1.
The total number of $$\pi $$ - bond electrons in the following structure is
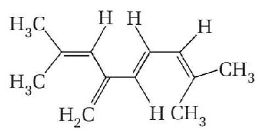
A
4
B
8
C
12
D
16
Answer :
8
2. Hyperconjugation is
A
$$\sigma - \pi $$ conjugation
B
noticed due to delocalisation of $$\sigma $$ and $$\pi $$ bonds
C
no bond resonance
D
all the above
Answer :
all the above
3.
Decreasing order of stability of following alkenes is
\[\begin{align}
& \left( \text{i} \right)C{{H}_{3}}-CH=C{{H}_{2}} \\
& \left( \text{ii} \right)C{{H}_{3}}-CH=CH-C{{H}_{3}} \\
& \left( \text{iii} \right)C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\mathop{-\,\,C=}}\,CH-C{{H}_{3}} \\
& \left( \text{iv} \right)C{{H}_{3}}\,\,\overset{\begin{smallmatrix}
\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-\,\,C\,=}}\,\overset{\begin{smallmatrix}
\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{\,\,\,C\,\,-}}\,\,\,C{{H}_{3}} \\
\end{align}\]
A
(i) > (ii) > (iii) > (iv)
B
(iv) > (iii) > (ii) > (i)
C
(iii) > (ii) > (i) > (iv)
D
(ii) > (iii) > (iv) > (i)
Answer :
(iv) > (iii) > (ii) > (i)
4.
The order of decreasing stability of the following carbanions is
$$\eqalign{
& \left( {\text{i}} \right){\left( {C{H_3}} \right)_3}{C^ - } \cr
& \left( {{\text{ii}}} \right){\left( {C{H_3}} \right)_2}C{H^ - } \cr
& \left( {{\text{iii}}} \right)C{H_3}CH_2^ - \cr
& \left( {{\text{iv}}} \right){C_6}{H_5}CH_2^ - \cr} $$
A
(i) > (ii) > (iii) > (iv)
B
(iv) > (iii) > (ii) > (i)
C
(iv) > (i) > (ii) > (iii)
D
(iii) > (ii) > (i) > (iv)
Answer :
(iv) > (iii) > (ii) > (i)
5.
Kl in acetone, undergoes $${S_N}2$$ reaction with each of $$P, Q, R$$ and $$S.$$ The rates of the reaction vary as

A
$$P > Q > R > S$$
B
$$S > P > R > Q$$
C
$$P > R > Q > S$$
D
$$R > P > S > Q$$
Answer :
$$S > P > R > Q$$
6. Heterolysis of a carbon-chlorine bond produces
A
two free radicals
B
two carbocations
C
one cation and one anion
D
two carbanions
Answer :
one cation and one anion
7. Point out the incorrect statement about resonance.
A
Resonance structures should have equal energy.
B
In resonance structures, the constituent atoms must be in the same position.
C
In resonance structures, there should not be same number of electron pairs.
D
Resonance structures should differ only in the location of electrons around the constituent atoms.
Answer :
In resonance structures, there should not be same number of electron pairs.
8.
The IUPAC name of the compound shown below is :
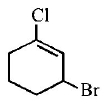
A
3 - bromo - 1- chlorocyclohexene
B
1 - bromo - 3 - chlorocyclohexene
C
2 - bromo - 6 - chlorocyclohex - 1 - ene
D
6 - bromo - 2 - chlorocyclohexene
Answer :
3 - bromo - 1- chlorocyclohexene
9. Dipole moment is shown by
A
1, 2-dichlorobenzene
B
$$trans$$ 2, 3-dichloro-2-butene
C
1, 4-chlorobenzene
D
$$trans$$ -1, 2-dinitroethene
Answer :
1, 2-dichlorobenzene
10.
The IUPAC name of the compound  is
is
A
1, 2-Propoxide
B
Propylene oxide
C
1, 2-Oxo propane
D
1, 2-Epoxy propane
Answer :
1, 2-Epoxy propane


