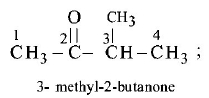61. Which of the following hydrocarbons has the lowest dipole moment ?
A


B
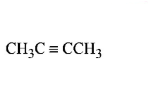
C
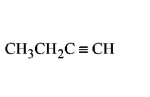
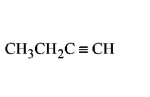
D
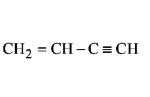
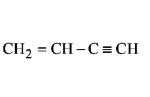
Answer :
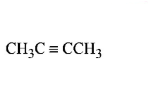
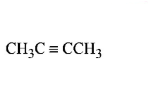
62.
Consider the following reactions,
\[\left( \text{i} \right)C{{H}_{2}}=CHCOOH\xrightarrow{\Delta }\] \[C{{H}_{2}}=C{{H}_{2}}\]
\[\left( \text{ii} \right)\] 
\[\left( \text{iii} \right)C{{H}_{3}}CH{{\left( COOH \right)}_{2}}\xrightarrow{\Delta }\] \[C{{H}_{3}}C{{H}_{2}}COOH\]
In which cases, parent compound loses its functional group in preference?
A
(i), (ii)
B
(i), (ii), (iii)
C
(ii), (iii)
D
(i), (iii)
Answer :
(i), (ii)
63.
IUPAC names of the given structures are

A
(i) hexane, (ii) 3-methylbutane
B
(i) isopentane, (ii) 2, 2-dimethylbutane
C
(i) 3-ethylbutane, (ii) isopentane
D
(i) 3-methylpentane, (ii) 2-methylbutane
Answer :
(i) 3-methylpentane, (ii) 2-methylbutane
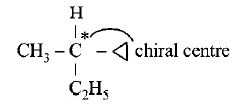
64. Amongst the following compounds, the optically active alkane having lowest molecular mass is
A


B
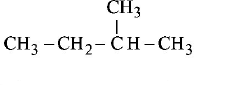
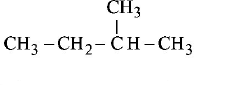
C


D
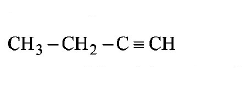
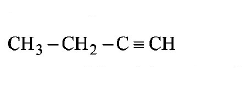
Answer :


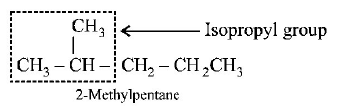
65. The compound which has one isopropyl group is :
A
2, 2, 3, 3 - tetramethylpentane
B
2, 2 - dimethylpentane
C
2, 2, 3 - trimethylpentane
D
2 - methylpentane
Answer :
2 - methylpentane
66. Which of the following alcohols on dehydration gives most stable carbocation?
A
\[C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
\,\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\mathop{-\,\,CH}}\,-C{{H}_{2}}OH\]
B
\[C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
\,\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,OH\]
C
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}OH\]
D
\[C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
\,\,\,\,OH
\end{smallmatrix}}{\mathop{-\,\,CH}}\,-C{{H}_{2}}C{{H}_{3}}\]
Answer :
\[C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
\,\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,OH\]
67.
The correct stability order for the following species is

A
(II) > (IV) > (I) > (III)
B
(I) > (II) > (III) > (IV)
C
(II) > (I) > (IV) > (III)
D
(I) > (III) > (II) > (IV)
Answer :
(I) > (III) > (II) > (IV)
68.
Consider the acidity of the carboxylic acids :
$$\eqalign{
& \left( {\text{i}} \right)PhCOOH \cr
& \left( {{\text{ii}}} \right)o{\text{ - }}N{O_2}{C_6}{H_4}COOH \cr
& \left( {{\text{iii}}} \right)p{\text{ - }}N{O_2}{C_6}{H_4}COOH \cr
& \left( {{\text{iv}}} \right)m{\text{ - }}N{O_2}{C_6}{H_4}COOH \cr} $$
Which of the following order is correct ?
A
(ii) > (iv) > (i) > (iii)
B
(ii) > (iv) > (iii) > (i)
C
(i) > (ii) > (iii) > (iv)
D
(ii) > (iii) > (iv) > (i)
Answer :
(ii) > (iii) > (iv) > (i)
69. The IUPAC name of $$C{H_3}COCH{\left( {C{H_3}} \right)_2}$$ is
A
2 - methyl - 3 - butanone
B
4 - methylisopropyl ketone
C
3 - methyl - 2 - butanone
D
Isopropylmethyl ketone
Answer :
3 - methyl - 2 - butanone
70. $$2.18\,g$$ of an organic compound containing sulphur produces $$1.02\,g$$ of $$BaS{O_4}.$$ The percentage of sulphur in the compound is
A
$$7.26\% $$
B
$$8.98\% $$
C
$$10\% $$
D
$$6.42\% $$
Answer :
$$6.42\% $$
 \[-COOH\] loses its preference
\[-COOH\] loses its preference \[\to C{{H}_{3}}C{{H}_{2}}COOH;-COOH\] does not lose its preference
\[\to C{{H}_{3}}C{{H}_{2}}COOH;-COOH\] does not lose its preference