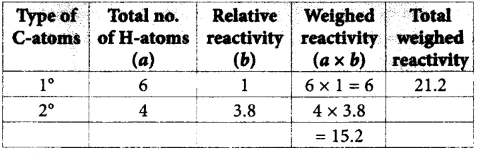
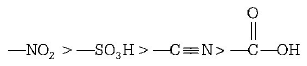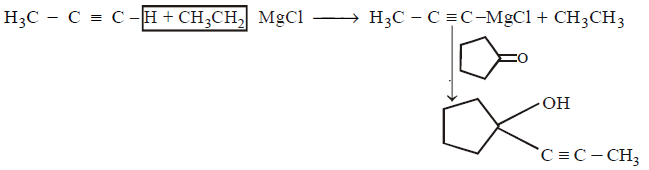221.
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\xrightarrow[h\upsilon ]{C{{l}_{2}}}\] \[\underset{\begin{smallmatrix}
\text{(monochlorination} \\
\text{products) }
\end{smallmatrix}}{\mathop{A+B\,\,\,\,\,\,\,\,\,\,\,\,}}\,\]
The approximate ratio of percentage yields of $$A$$ and $$B$$ formed in the above reaction is
A
50 : 50
B
72 : 28
C
45 : 55
D
60 : 40
Answer :
72 : 28
222. Which of the following will form alkynide ?
A
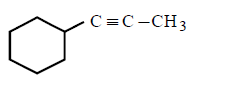
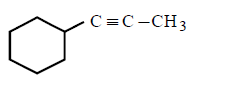
B
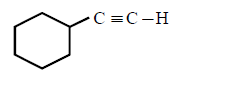
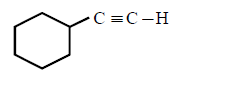
C
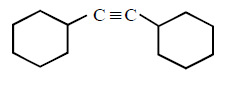

D
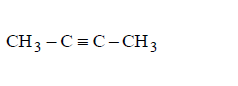
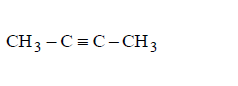
Answer :
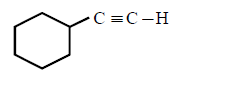
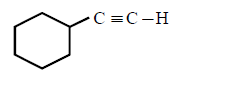
223. On mixing a certain alkane with chlorine and irradiating it with ultraviolet light, it forms only one monochloroalkane. This alkane could be
A
pentane
B
isopentane
C
neopentane
D
propane
Answer :
neopentane
224. Some $$meta$$ - directing substituents in aromatic substitution are given. Which one is most deactivating?
A
$$ - C \equiv N$$
B
$$ - S{O_3}H$$
C
$$ - COOH$$
D
$$ - N{O_2}$$
Answer :
$$ - N{O_2}$$
225. Which of the following reactions does not show the acidic nature of ethyne?
A
Acetylene reacts with sodamide to form sodium acetylides.
B
When passed through ammoniacal cuprous chloride solution, a red precipitate is formed.
C
Acetylenereacts with chlorine in the dark to form di or tetrachlorides.
D
Acetylene when passed through ammoniacal silver nitrate gives a white precipitate.
Answer :
Acetylenereacts with chlorine in the dark to form di or tetrachlorides.
226.
Match the column I with column II to identify the products of oxidation of alkanes and mark the appropriate choice.
| Column I | Column II | ||
|---|---|---|---|
| a. | \[{\left( {C{H_3}} \right)_3}CH + 2 {O_2}\xrightarrow[{\left[ O \right]}]{{KMn {O_4}}}\] | 1. | $$HCOOH + {H_2}O$$ |
| b. | \[2C{H_4} + {O_2}\xrightarrow[{100\,atm}] {{Cu/523\,K}}\] | 2. | $${\left( {C{H_3}} \right)_3}COH$$ |
| c. | \[C{{H}_{4}}+{{O}_{2}}\xrightarrow[\Delta ]{M{{o}_{2}}{{O}_{3}}}\] | 3. | $$2C{H_3}OH$$ |
| d. | \[C{{H}_{4}}+\frac{3}{2}{{O}_ {2}}\xrightarrow{{{\left( C{{H}_{3}}COO \right)}_ {2}}Mn}\] | 4. | $$HCHO + {H_2}O$$ |
A
a - 1, b - 2, c - 3, d - 4
B
a - 2, b - 3, c - 4, d - 1
C
a - 4, b - 2, c - 3, d - 1
D
a - 3, b - 1, c - 2, d - 4
Answer :
a - 2, b - 3, c - 4, d - 1
227.
The major product of the following reaction
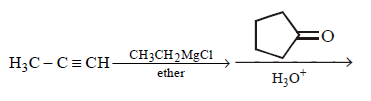
A
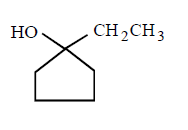
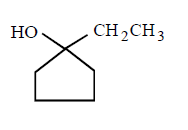
B
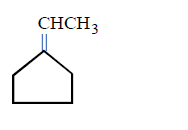

C
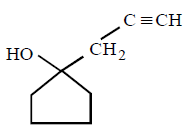

D
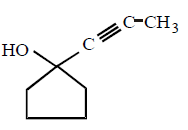
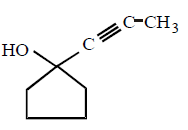
Answer :
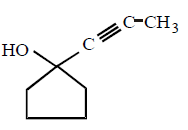
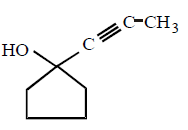
228. At 300 K and 1 atm, 15 mL of a gaseous hydrocarbon requires $$375 mL$$ air containing 20% $${O_2}$$ by volume for complete combustion. After combustion the gases occupy $$330 mL.$$ Assuming that the water formed is in liquid form and the volumes were measured at the same temperature and pressure, the formula of the hydrocarbon is :
A
$${C_4}{H_8}$$
B
$${C_4}{H_{10}}$$
C
$${C_3}{H_6}$$
D
$${C_3}{H_8}$$
Answer :
$${C_3}{H_8}$$
229. Which of the following compounds has the lowest boiling point?
A
$$C{H_3}C{H_2}C{H_2}C{H_2}C{H_3}$$
B
$$C{H_3}CH = CH - C{H_2}C{H_3}$$
C
$$C{H_3}CH = CH - CH = C{H_2}$$
D
$$C{H_3}C{H_2}C{H_2}C{H_3}$$
Answer :
$$C{H_3}C{H_2}C{H_2}C{H_3}$$
230. Ethane is formed during the formation of chloromethane by chlorination of methane because
A
higher members of the hydrocarbons are generally formed during reactions
B
two methyl free radicals may combine during chlorination to give ethane
C
two chloromethane molecules react to form ethane
D
chlorine free radical reacts with methane to give ethane
Answer :
two methyl free radicals may combine during chlorination to give ethane