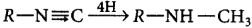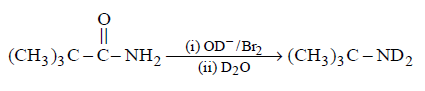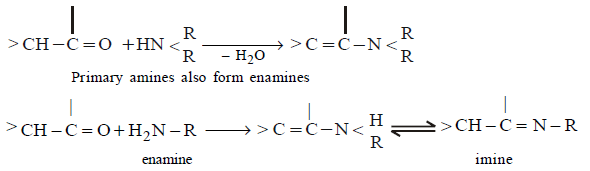161. Secondary amines could be prepared by
A
reduction of nitriles
B
Hoffmann bromamide reaction
C
reduction of isonitrile
D
all of these
Answer :
reduction of isonitrile
162.
Match the compounds in List $$I$$ with their nature from List $$II,$$ as seen in aqueous medium
$$\eqalign{
& \,\,\,\,\,\,\,\,\,{\text{List I }}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{List II}} \cr
& {\text{I}}{\text{. Acetamide }}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{A}}{\text{. Acidic}} \cr
& {\text{II}}{\text{. Benzonitrile }}\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{B}}{\text{. Basic}} \cr
& {\text{III}}{\text{. Triethylamine }}\,\,\,\,\,\,{\text{C}}{\text{. Neutral}} \cr
& {\text{IV}}{\text{. Phenol}} \cr} $$
A
I - C; II - C; III - B; IV - A
B
I - B; II - C; III - C; IV - A
C
I - C; II - B; III - B; IV - C
D
I - A; II - A; III - C; IV - B
Answer :
I - C; II - C; III - B; IV - A
163.
In the reaction
 \[C{{H}_{2}}CN\xrightarrow[NaN{{H}_{2}},\,N{{H}_{3}},\,-{{80}^{\circ }}C]{C{{H}_{3}}Br}\]
\[C{{H}_{2}}CN\xrightarrow[NaN{{H}_{2}},\,N{{H}_{3}},\,-{{80}^{\circ }}C]{C{{H}_{3}}Br}\]
the products obtained are
A


B


C


D
Answer :


164. Carbylamine test is performed in alcoholic \[KOH\] by heating a mixture of :
A
chloroform and silver powder
B
trihalogenatedmethane and a primary amine
C
an alkyl halide anda primary amine
D
an alkyl cyanide and a primary amine
Answer :
trihalogenatedmethane and a primary amine
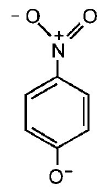
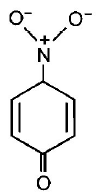
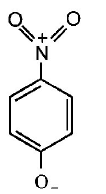
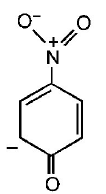
165. The most unlikely representation of resonance structures of $$p $$ - nitrophenoxide ion is
A

B

C

D

Answer :


166.
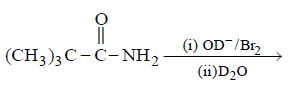 Product $$P$$ is
Product $$P$$ is
A
\[{{\left( C{{H}_{3}} \right)}_{3}}CN{{H}_{2}}\]
B
\[{{\left( C{{H}_{3}} \right)}_{3}}CNHD\]
C
\[{{\left( C{{H}_{3}} \right)}_{3}}CN{{D}_{2}}\]
D
\[\text{no reaction}\]
Answer :
\[{{\left( C{{H}_{3}} \right)}_{3}}CN{{D}_{2}}\]
167. Primary amines react with benzoyl chloride to give
A
benzamides
B
ethanamides
C
imides
D
imines
Answer :
benzamides
168. Which of the following amines will react with cyclohexanone to give enamine ?
A
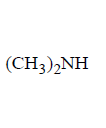
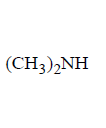
B
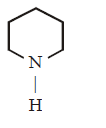

C
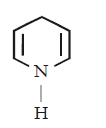

D
Answer :
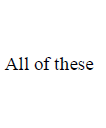
169. Aniline reacts with phosgene and $$KOH$$ to form
A


B
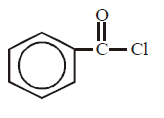

C
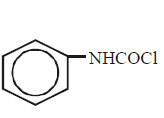

D


Answer :


170.
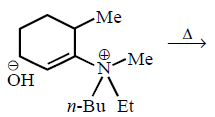
The alkene formed as a major product in the above elimination reaction is
A
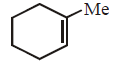

B
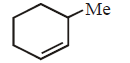

C
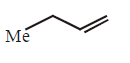

D
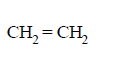
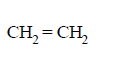
Answer :