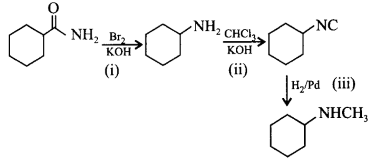
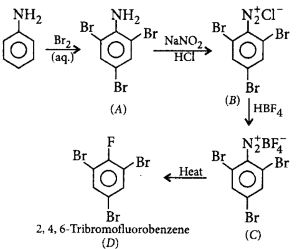
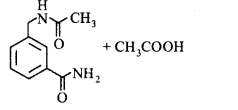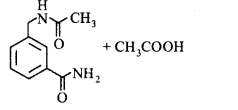171.
Which of the following methods can be used to carry out the following conversions?
| (i) | (ii) | (iii) | |
|---|---|---|---|
| (a) | $$B{r_2}/KOH$$ | $$CHC{l_3}/KOH$$ | $${H_2}/Pd$$ |
| (b) | $$KCN$$ | $${H_2}/Pd$$ | $$Sn/HCl$$ |
| (c) | $$CuCN$$ | $${H_2}O/{H^ + }$$ | $${H_2}/Pd$$ |
| (d) | $$HN{O_3}/{H_2}S{O_4}$$ | $${\left( {C{H_3}CO} \right)_2}O$$ | $$Fe/HCl$$ |
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(a)
172.
The correct order of increasing reactivity of $$C-X$$ bond towards nucleophile in the following compounds is

A
I < II < IV < III
B
II < III < I < IV
C
IV < III < I < II
D
III < II < I < IV
Answer :
I < II < IV < III
173. A compound with molecular mass 180 is acylated with $$C{H_3}COCl$$ to get a compound with molecular mass 390. The number of amino groups present per molecule of the former compound is :
A
2
B
5
C
4
D
6
Answer :
5
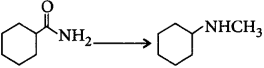
174. In the chemical reaction, $$C{H_3}C{H_2}N{H_2} + CHC{l_3} + 3KOH$$ $$ \to \left( A \right) + \left( B \right) + 3{H_2}O,$$ the compounds $$\left( A \right)$$ and $$\left( B \right)$$ are respectively
A
$${C_2}{H_5}NC\,{\text{and}}\,3KCl$$
B
$${C_2}{H_5}CN\,{\text{and}}\,3KCl$$
C
$$C{H_3}C{H_2}CON{H_2}\,{\text{and}}\,3KCl$$
D
$${C_2}{H_5}NC\,{\text{and}}\,{K_2}C{O_3}$$
Answer :
$${C_2}{H_5}NC\,{\text{and}}\,3KCl$$
175. Which of the following is the strongest base ?
A
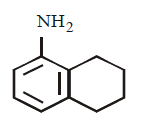

B
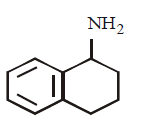

C
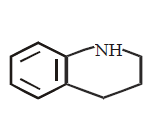

D

Answer :


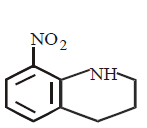
176.
The product $$'D'$$ in the following sequence of reactions is
 \[\xrightarrow[\left( aq. \right)]{B{{r}_{2}}}A\xrightarrow[HCl]{NaN{{O}_{2}}}B\xrightarrow{HB{{F}_{4}}}\] \[C\xrightarrow{\text{heat}}D\]
\[\xrightarrow[\left( aq. \right)]{B{{r}_{2}}}A\xrightarrow[HCl]{NaN{{O}_{2}}}B\xrightarrow{HB{{F}_{4}}}\] \[C\xrightarrow{\text{heat}}D\]
A
2, 4, 6 - tribromofluorobenzene
B
fluorobenzene
C
$$p$$ - bromofluorobenzene
D
tribromobenzene
Answer :
2, 4, 6 - tribromofluorobenzene
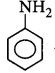
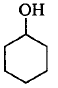
177. Which of the following compounds is the weakest $${\rm{Br\ddot onsted}}$$ base?
A
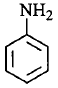

B
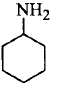

C
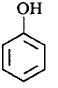

D

Answer :


178. Acetanilide on nitration followed by alkaline hydrolysis mainly gives
A
$$o$$ - Nitroacetanilide
B
$$p$$ - Nitroaniline
C
$$m$$ - Nitroaniline
D
2, 4, 6 - Trinitroaniline
Answer :
$$p$$ - Nitroaniline
179.
Match the compounds given in column I with column II and mark the appropriate choice.
| Column I | Column II | ||
|---|---|---|---|
| a. | Benzenesulphonyl chloride | 1. | Zwitter ion |
| b. | Sulphanilic acid | 2. | Hinsberg's reagent |
| c. | Alkyldiazonium salts | 3. | Dyes |
| d. | Aryldiazonium salts | 4. | Conversion to alcohols |
A
a - 4, b - 3, c - 1, d - 2
B
a - 2, b - 4, c - 3, d - 1
C
a - 2, b - 1, c - 4, d - 3
D
a - 2, b - 3, c - 4, d - 1
Answer :
a - 2, b - 1, c - 4, d - 3
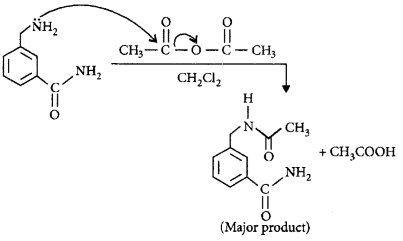
180.
In the reaction shown below, the major product(s) formed is/are
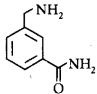 \[\xrightarrow[C{{H}_{2}}C{{l}_{2}}]{\text{acetic}\,\text{anhydride}}\text{product}\left( \text{S} \right)\]
\[\xrightarrow[C{{H}_{2}}C{{l}_{2}}]{\text{acetic}\,\text{anhydride}}\text{product}\left( \text{S} \right)\]
A
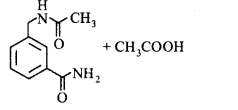
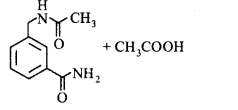
B
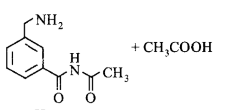

C
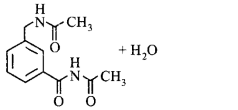

D

Answer :