151. What will be the wavelength of an electron moving with $$\frac{1}{{10}}th$$ of velocity of light ?
A
$$2.43 \times {10^{ - 11}}\,m$$
B
$$243 \times {10^{ - 11}}\,m$$
C
$$0.243\,m$$
D
$$2.43 \times {10^{ - 4}}\,m$$
Answer :
$$2.43 \times {10^{ - 11}}\,m$$
152. The wavelength of a spectral line for an electronic transition is inversely related to :
A
the number of electrons undergoing the transition
B
the nuclear charge of the atom
C
the difference in the energy of the energy levels involved in the transition
D
the velocity of the electron undergoing the transition.
Answer :
the difference in the energy of the energy levels involved in the transition
153. The uncertainty in the position of an electron $$\left( {mass = 9.1 \times {{10}^{ - 28}}g} \right)$$ moving with a velocity of $$3.0 \times {10^4}cm\,{s^{ - 1}}$$ accurate upto $$0.011\% $$ will be
A
$$1.92\,cm$$
B
$$7.68\,cm$$
C
$$0.175\,cm$$
D
$$3.84\,cm$$
Answer :
$$0.175\,cm$$
154. The longest wavelength doublet absorption transition is observed at 589 and 589.6$$\,nm.$$ Energy difference between two excited states is
A
$$3.31 \times {10^{ - 22}}kJ$$
B
$$3.31 \times {10^{ - 22}}J$$
C
$$2.98 \times {10^{ - 21}}J$$
D
$$3.0 \times {10^{ - 21}}kJ$$
Answer :
$$3.31 \times {10^{ - 22}}J$$
155. If the de-Broglie wavelength of a particle of mass $$m$$ is 100 times its velocity, then its value in terms of its mass $$(m)$$ and Planck’s constant $$(h)$$ is
A
$$\frac{1}{{10}}\sqrt {\frac{m}{h}} $$
B
$$10\sqrt {\frac{h}{m}} $$
C
$$\frac{1}{{10}}\sqrt {\frac{h}{m}} $$
D
$$10\sqrt {\frac{m}{h}} $$
Answer :
$$10\sqrt {\frac{h}{m}} $$
156. The group having isoelectronic species is :
A
$${O^{2 - }},{F^ - },N{a^ + },M{g^{2 + }}$$
B
$${O^ - },{F^ - },Na,M{g^ + }$$
C
$${O^{2 - }},{F^ - },Na,M{g^{2 + }}$$
D
$${O^ - },{F^ - },N{a^ + },M{g^{2 + }}$$
Answer :
$${O^{2 - }},{F^ - },N{a^ + },M{g^{2 + }}$$
157. What should be the kinetic energy and total energy of the electron present in hydrogen atom, if its potential energy is $$ - 5.02\,eV?$$
A
$$ - 2.51\,eV,\, + 2.51\,eV$$
B
$$ + 0.251\,eV,\, - 0.251\,eV$$
C
$$ - 2.51\,eV, - 2.51\,eV$$
D
$$ + 2.51\,eV, + 2.51\,eV$$
Answer :
$$ - 2.51\,eV,\, + 2.51\,eV$$
158. If the radius of first Bohr orbit is $$x$$ $$pm,$$ then the radius of the third orbit would be
A
$$\left( {3 \times x} \right)pm$$
B
$$\left( {6 \times x} \right)pm$$
C
$$\left( {\frac{1}{2} \times x} \right)pm$$
D
$$\left( {9 \times x} \right)pm$$
Answer :
$$\left( {9 \times x} \right)pm$$
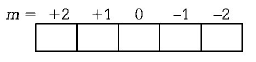
159. The following quantum numbers are possible for how many orbital$$(s)$$ $$n = 3,l = 2$$ and $$m = + 2?$$
A
1
B
2
C
3
D
4
Answer :
1
160. What will be the uncertainty in velocity of a bullet with a mass of $$10\,g$$ whose position is known with $$ \pm 0.01\,mm?$$
A
$$5.275 \times {10^{ - 33}}\,m\,{s^{ - 1}}$$
B
$$5.275 \times {10^{ - 25}}\,m\,{s^{ - 1}}$$
C
$$5.275 \times {10^{ - 5}}\,m\,{s^{ - 1}}$$
D
$$5.275 \times {10^{ - 28}}\,m\,{s^{ - 1}}$$
Answer :
$$5.275 \times {10^{ - 28}}\,m\,{s^{ - 1}}$$