161. The wavelength associated with a golf ball weighing $$200g$$ and moving at a speed of $$5\,m/h$$ is of the order
A
$${10^{ - 10}}m$$
B
$${10^{ - 20}}m$$
C
$${10^{ - 30}}m$$
D
$${10^{ - 40}}m$$
Answer :
$${10^{ - 10}}m$$
162.
Observe the given boundary surface diagrams of two orbitals $$I$$ and $$II$$ and choose the correct option.
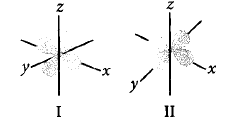
A
$$I{\text{ - }}{d_{{x^2} - {y^2}}},II{\text{ - }}{d_{yz}}$$
B
$$I{\text{ - }}{d_{yz}},II{\text{ - }}{d_{{x^2} - {y^2}}}$$
C
$$I{\text{ - }}{d_{xz}},II{\text{ - }}{d_{{z^2}}}$$
D
$$I{\text{ - }}{d_{xy}},II{\text{ - }}{d_{xz}}$$
Answer :
$$I{\text{ - }}{d_{yz}},II{\text{ - }}{d_{{x^2} - {y^2}}}$$
163. Select the incorrect graph for velocity of $${e^ - }$$ in an orbit vs. $$Z,\frac{1}{n}$$ and $$n$$ :
A
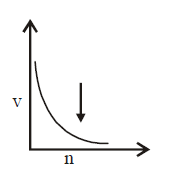

B
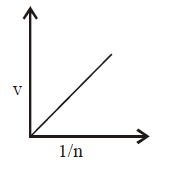

C
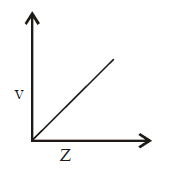

D
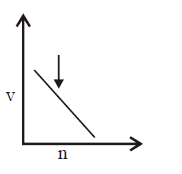

Answer :


164. What will be the energy of a photon which corresponds to the wavelength of $$0.50\,\mathop {\text{A}}\limits^{\text{o}} ?$$
A
$$3.98 \times {10^{ - 15}}J$$
B
$$3 \times {10^{15}}J$$
C
$$3.9 \times {10^8}J$$
D
$$3 \times {10^{ - 34}}J$$
Answer :
$$3.98 \times {10^{ - 15}}J$$
165. The frequency of radiation emitted when the electron falls from $$n = 4$$ to $$n = 1$$ in a hydrogen atom will be (Given ionisation energy of $$H = 2.18 \times {10^{ - 18}}J\,ato{m^{ - 1}}$$ and $$h = 6.625 \times {10^{ - 34}}Js$$ )
A
$$1.54 \times {10^{15}}{s^{ - 1}}$$
B
$$1.03 \times {10^{15}}{s^{ - 1}}$$
C
$$3.08 \times {10^{15}}{s^{ - 1}}$$
D
$$2.00 \times {10^{15}}{s^{ - 1}}$$
Answer :
$$3.08 \times {10^{15}}{s^{ - 1}}$$
166. In hydrogen atom, energy of first excited state is $$ - 3.4\,eV.$$ Then, $$KE$$ of same orbit of hydrogen atom is
A
$$ + 3.4\,eV$$
B
$$ + 6.8\,eV$$
C
$$ - 13.6\,eV$$
D
$$ + 13.6\,eV$$
Answer :
$$ + 3.4\,eV$$
167. Excited hydrogen atom emits light in the ultraviolet region at $$2.47 \times {10^{15}}Hz.$$ With this frequency, the energy of a single photon is : $$\left( {h = 6.63 \times {{10}^{ - 34}}Js} \right)$$
A
$$8.041 \times {10^{ - 40}}J$$
B
$$2.680 \times {10^{ - 19}}J$$
C
$$1.640 \times {10^{ - 18}}J$$
D
$$6.111 \times {10^{ - 17}}J$$
Answer :
$$1.640 \times {10^{ - 18}}J$$
168. Let $${m_p}$$ be the mass of a proton, $${m_n}$$ that of a neutron, $${M_1}$$ that of a $$_{10}^{20}Ne$$ nucleus and $${M_2}$$ that of a $$_{20}^{40}Ca$$ nucleus. Then
A
$${M_2} = 2{M_1}$$
B
$${M_1} < 10\left( {{m_p} + {m_n}} \right)$$
C
$${M_2} > 2{M_1}$$
D
$${M_1} = {M_2}$$
Answer :
$${M_2} = 2{M_1}$$
169. Given that the abundances of isotopes $${}^{54}Fe,\,{}^{56}Fe$$ and $${}^{57}Fe$$ are $$5\% ,90\% $$ and $$5\% ,$$ respectively, the atomic mass of $$Fe$$ is
A
55.85
B
55.95
C
55.75
D
56.05
Answer :
55.95
170. The orbital diagram in which the Aufbau principle is violated is
A
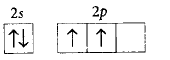

B
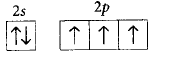

C
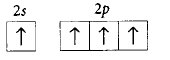

D
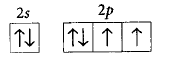

Answer :

