171. The orbitals are called degenerate when
A
they have the same wave functions
B
they have the same wave functions but different energies
C
they have different wave functions but same energy
D
they have the same energy
Answer :
they have the same energy
172. The measurement of the electron position is associated with an uncertainty in momentum, which is equal to $$1 \times {10^{ - 18}}gcm\,\,{s^{ - 1}}.$$ The uncertainty in electron velocity is ( mass of an electron is $$9 \times {10^{ - 28}}g$$ )
A
$$1 \times {10^9}cm\,\,{s^{ - 1}}$$
B
$$1 \times {10^6}cm\,\,{s^{ - 1}}$$
C
$$1 \times {10^5}cm\,\,{s^{ - 1}}$$
D
$$1 \times {10^{11}}cm\,\,{s^{ - 1}}$$
Answer :
$$1 \times {10^9}cm\,\,{s^{ - 1}}$$
173. According to Bohr's theory, the angular momentum of an electron in 5th orbit is
A
$$\frac{{10h}}{\pi }$$
B
$$\frac{{25h}}{\pi }$$
C
$$\frac{{1.5h}}{\pi }$$
D
$$\frac{{2.5h}}{\pi }$$
Answer :
$$\frac{{2.5h}}{\pi }$$
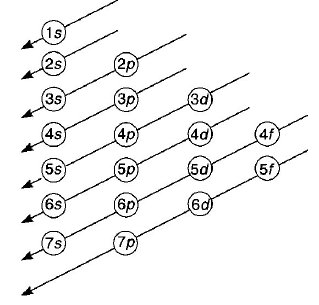
174. The order of filling of electrons in the orbitals of an atom will be
A
$$3d,4s,4p,4d,5s$$
B
$$4s,3d,4p,5s,4d$$
C
$$5s,4p,3d,4d,5s$$
D
$$3d,4p,4s,4d,5s$$
Answer :
$$4s,3d,4p,5s,4d$$
175. According to Bohr's theory, the angular momentum of an electron in 5th orbit is
A
$$\frac{{10h}}{\pi }$$
B
$$\frac{{2.5h}}{\pi }$$
C
$$\frac{{25h}}{\pi }$$
D
$$\frac{{1.0h}}{\pi }$$
Answer :
$$\frac{{2.5h}}{\pi }$$
176. The wave number of the first emission line in the Balmer series of $$H$$-Spectrum is : ( $$R =$$ Rydberg constant ) :
A
$$\frac{5}{{36}}R$$
B
$$\frac{9}{{400}}R$$
C
$$\frac{7}{6}R$$
D
$$\frac{3}{4}R$$
Answer :
$$\frac{5}{{36}}R$$
177. According to law of photochemical equivalence the energy absorbed ( in $$ergs/mole$$ ) is given as $$\left( {h = 6.62 \times {{10}^{ - 27}}ergs,\,c = 3 \times {{10}^{10}}cm\,\,{s^{ - 1}},{N_A} = 6.02 \times {{10}^{23}}mo{l^{ - 1}}} \right)$$
A
$$\frac{{1.956 \times {{10}^{16}}}}{\lambda }$$
B
$$\frac{{1.19 \times {{10}^8}}}{\lambda }$$
C
$$\frac{{2.859 \times {{10}^5}}}{\lambda }$$
D
$$\frac{{2.859 \times {{10}^{16}}}}{\lambda }$$
Answer :
$$\frac{{1.19 \times {{10}^8}}}{\lambda }$$
178. The orbital angular momentum of an electron in $$2s$$ orbital is:
A
$$ + \frac{1}{2}.\frac{h}{{2\pi }}$$
B
Zero
C
$$\frac{h}{{2\pi }}$$
D
$$\sqrt 2 .\frac{h}{{2\pi }}$$
Answer :
Zero
179. The radius of the stationary state which is also called Bohr radius is given by the expression $${r_n} = {n^2}{a_0}$$ where the value of $${a_0}$$ is
A
52.9$$\,pm$$
B
5.29$$\,pm$$
C
529$$\,pm$$
D
0.529$$\,pm$$
Answer :
52.9$$\,pm$$
180. Given, the mass of electron is $$9.11 \times {10^{ - 31}}kg,$$ Planck’s constant is $$6.626 \times {10^{ - 34}}Js,$$ the uncertainty involved in the measurement of velocity within a distance of $$0.1\mathop {\text{A}}\limits^{\text{o}} $$ is
A
$$5.79 \times {10^6}m{s^{ - 1}}$$
B
$$5.79 \times {10^7}m{s^{ - 1}}$$
C
$$5.79 \times {10^8}m{s^{ - 1}}$$
D
$$5.79 \times {10^5}m{s^{ - 1}}$$
Answer :
$$5.79 \times {10^6}m{s^{ - 1}}$$