181. A gas absorbs a photon of $$355 \,nm$$ and emits at two wavelengths. If one of the emissions is at $$680\, nm,$$ the other is at :
A
1035 $$nm$$
B
325 $$nm$$
C
743 $$nm$$
D
518 $$nm$$
Answer :
743 $$nm$$
182. The de-Broglie wavelength of a particle with mass $$1 g$$ and velocity $$100 m/s$$ is
A
$$6.63 \times {10^{ - 33}}m$$
B
$$6.63 \times {10^{ - 34}}m$$
C
$$6.63 \times {10^{ - 35}}m$$
D
$$6.65 \times {10^{ - 36}}m$$
Answer :
$$6.63 \times {10^{ - 33}}m$$
183. The energy of an electron in first Bohr orbit of $$H$$-atom is $$ - 13.6\,eV.$$ The energy value of electron in the excited state of $$L{i^{2 + }}$$ is :
A
$$ - 27.2\,eV$$
B
$$30.6\,eV$$
C
$$ - 30.6\,eV$$
D
$$27.2\,eV$$
Answer :
$$ - 30.6\,eV$$
184. Table-tennis ball has a mass $$10\,g$$ and a speed of $$100\,m/s.$$ If speed can be measured within an accuracy of $$10\% ,$$ what will be the uncertainty in speed and position respectively ?
A
$$10\,m/\sec ,4 \times {10^{ - 33}}\,m$$
B
$$10\,m/\sec ,5.27 \times {10^{ - 34}}\,m$$
C
$$0.1\,m/\sec ,5 \times {10^{ - 34}}\,m$$
D
$${\text{None of these}}$$
Answer :
$$10\,m/\sec ,5.27 \times {10^{ - 34}}\,m$$
185. The isotopes of hydrogen are:
A
Tritium and protium only
B
Protium and deuterium only
C
Protium, deuterium and tritium
D
Deuterium and tritium only
Answer :
Protium, deuterium and tritium
186.
In a multi-electron atom, which of the following orbitals described by the three quantum members will have the same energy in the absence of magnetic and electric fields?
(A) $$n = 1,\,l = 0,\,m = 0$$
(B) $$n = 2,\,l = 0,\,m = 0$$
(C) $$n = 2,\,l = 1,\,m = 1$$
(D) $$n = 3,\,l = 2,\,m = 1$$
(E) $$n = 3,\,l = 2,\,m = 0$$
A
(D) and (E)
B
(C) and (D)
C
(B) and (C)
D
(A) and (B)
Answer :
(D) and (E)
187. An orbital is described with the help of a wave function. Since many wave functions are possible for an electron, there are many atomic orbitals. When atom is placed in a magnetic field the possible number of orientations for an orbital of azimuthal quantum number 3 is
A
three
B
two
C
five
D
seven
Answer :
seven
188. Which of the following configurations represents a noble gas ?
A
$$1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^{10}}4{s^2}4{p^6}4{d^{10}}5{s^2}$$
B
$$1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^{10}}4{s^2}4{f^{14}}5{s^2}$$
C
$$1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^{10}}4{s^2}4{p^6}4{d^{10}}5{s^2}5{p^6}$$
D
$$1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^{10}}4{s^2}4{p^6}4{d^{10}}5{s^2}5{p^3}$$
Answer :
$$1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^{10}}4{s^2}4{p^6}4{d^{10}}5{s^2}5{p^6}$$
189.
The orbital diagram in which the Aufbau principle is violated is:
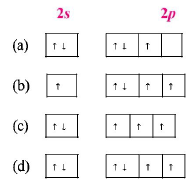
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(b)
190. Which one of the following sets of ions represents a collection of isoelectronic species?
A
$${N^{3 - }},{O^{2 - }},{F^ - },{S^{2 - }}$$
B
$$L{i^ + },N{a^ + },M{g^{2 + }},C{a^{2 + }}$$
C
$${K^ + },C{l^ - },C{a^{2 + }},S{c^{3 + }}$$
D
$$B{a^{2 + }},S{r^{2 + }},{K^ + },C{a^{2 + }}$$
Answer :
$${K^ + },C{l^ - },C{a^{2 + }},S{c^{3 + }}$$