241.
Which of the following statements is correct about the given Daniell cell?
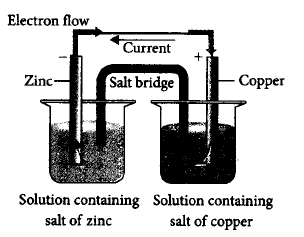
A
This cell converts the electrical energy liberated during the redox reaction to chemical energy.
B
This cell has an electrical potential greater than $$1.1\,V$$ when concentration of $$Z{n^{2 + }}$$ and $$C{u^{2 + }}$$ ions is unity $$\left( {1\,mol\,d{m^{ - 3}}} \right).$$
C
In this cell, copper is acting as cathode and zinc is acting as anode.
D
Redox reaction occurring in this cell is $$C{u_{\left( s \right)}} + Zn_{\left( {aq} \right)}^{2 + } \to Cu_{\left( {aq} \right)}^{2 + } + Z{n_{\left( s \right)}}$$
Answer :
In this cell, copper is acting as cathode and zinc is acting as anode.
242. For the electrochemical cell, $$M\left| {{M^ + }} \right|\left| {{X^ - }} \right|X,{E^o}{M^ + }/M = 0.44V\,{\text{and}}\,{E^ \circ }\left( {X/X} \right) = 0.33V.$$ From this data one can deduce that
A
$$M + X \to {M^ + } + {X^ - }$$ is the spontaneous reaction
B
$${M^ + } + {X^ - } \to M + X$$ is the spontaneous reaction
C
$${E_{cell}} = 0.77V$$
D
$${E_{cell}} = - 0.77V$$
Answer :
$${M^ + } + {X^ - } \to M + X$$ is the spontaneous reaction
243. During the electrolysis of molten sodium chloride, the time required to produce $$0.10\,mol$$ of chlorine gas using a current of 3 amperes is
A
55 minutes
B
110 minutes
C
220 minutes
D
330 minutes
Answer :
110 minutes
244. The Nernst equation $$E = {E^ \circ } - \frac{{RT}}{{nF}}\,\ln \,Q$$ indicates that the $$Q$$ will be equal to equilibrium constant $${K_c}$$ when :
A
$$E = {E^ \circ }$$
B
$$\frac{{RT}}{{nF}} = 1$$
C
$$E = {\text{zero}}$$
D
$${E^ \circ } = 1$$
Answer :
$$E = {\text{zero}}$$
245.
Following cell has $$EMF\,0.7995\,V.$$
$$Pt\left| {{H_2}\left( {1\,atm} \right)} \right|\left. {HN{O_3}\left( {1\,M} \right)} \right|\left| {AgN{O_3}\left( {1\,M} \right)} \right|Ag$$
If we add enough $$KCl$$ to the $$Ag$$ cell so that the final $$C{l^ - }$$ is $$1M.$$ Now the measured emf of the cell is $$0.222V.$$ The $${K_{sp}}$$ of $$AgCl$$ would be-
A
$$1 \times {10^{ - 9.8}}$$
B
$$1 \times {10^{ - 19.6}}$$
C
$$2 \times {10^{ - 10}}$$
D
$$2.64 \times {10^{ - 14}}$$
Answer :
$$1 \times {10^{ - 9.8}}$$
246. Without losing its concentration $$ZnC{l_2}$$ solution cannot be kept in contact with
A
$$Au$$
B
$$Al$$
C
$$Pb$$
D
$$Ag$$
Answer :
$$Al$$
247. Which of the following statement is correct ?
A
Cathode is $$–ve$$ terminal in both, glavanic and electrolytic cells.
B
Anode is $$+ve$$ terminal in both, galvanic and electrolytic cells.
C
Cathode and anode are $$–ve$$ terminal in electrolytic and galvanic cell.
D
Cathode and anode are $$+ve$$ terminal in electrolytic and galvanic cell.
Answer :
Cathode and anode are $$–ve$$ terminal in electrolytic and galvanic cell.
248. The overall reaction of a hydrogen-oxygen fuel cell is
A
$$2{H_{2\left( g \right)}} + {O_{2\left( g \right)}} \to 2{H_2}{O_{\left( l \right)}}$$
B
$$2{H_{2\left( g \right)}} + 4OH_{\left( {aq} \right)}^ - \to 4{H_2}{O_{\left( l \right)}} + 4{e^ - }$$
C
$${O_{2\left( g \right)}} + 2{H_2}{O_{\left( l \right)}} + 4{e^ - } \to 4OH_{\left( {aq} \right)}^ - $$
D
$$4OH_{\left( {aq} \right)}^ - + 4{e^ - } \to 2{H_2}{O_{\left( l \right)}}$$
Answer :
$$2{H_{2\left( g \right)}} + {O_{2\left( g \right)}} \to 2{H_2}{O_{\left( l \right)}}$$
249. Which of the following chemical reactions depict the oxidizing beahviour of $${H_2}S{O_4}$$ ?
A
$$NaCl + {H_2}S{O_4} \to NaHS{O_4} + HCl$$
B
$$2PC{l_5} + {H_2}S{O_4} \to 2POC{l_3} + 2HCl + S{O_2}C{l_2}$$
C
$$2HI + {H_2}S{O_4} \to {I_2} + S{O_2} + 2{H_2}O$$
D
$$Ca{\left( {OH} \right)_2} + {H_2}S{O_4} \to CaS{O_4} + 2{H_2}O$$
Answer :
$$2HI + {H_2}S{O_4} \to {I_2} + S{O_2} + 2{H_2}O$$
250. Standard reduction electrode potentials of three metals $$A, B$$ & $$C$$ are respectively $$+ 0.5 V, -3.0 V$$ & $$-1.2 V.$$ The reducing powers of these metals are
A
$$A > B > C$$
B
$$C > B > A$$
C
$$A > C > B$$
D
$$B > C > A$$
Answer :
$$B > C > A$$