321.
The formal potential of $$\frac{{F{e^{3 + }}}}{{F{e^{2 + }}}}$$ in a sulphuric acid and phosphoric acid mixture $$\left( {{E^ \circ } = + 0.61\,V} \right)$$ is much lower than the standard potential $$\left( {{E^ \circ } = + 0.77\,V} \right).$$
This is due to
(i) formation of the species $${\left[ {FeHP{O_4}} \right]^ + }$$
(ii) lowering of potential upon complexation
(iii) formation of the species $${\left[ {FeS{O_4}} \right]^ + }$$
(iv) high acidity of the medium
A
(i) and (ii) only
B
(i), (ii) and (iv) only
C
(iii) only
D
all of these
Answer :
(i) and (ii) only
322.
A cell is set up as shown in the figure. It is observed that $$EMF$$ of the cell comes out to be 2.36 $$V.$$ Which of the given statements is not correct about the cell?
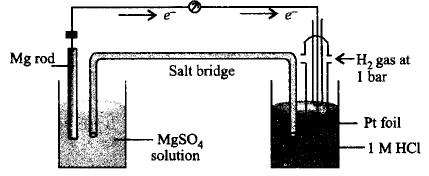
A
Reduction takes place at magnesium electrode and oxidation at $$SHE.$$
B
Oxidation takes place at magnesium electrode and reduction at $$SHE.$$
C
Standard electrode potential for $$\frac{{M{g^{2 + }}}}{{Mg}}$$ will be - 2.36$$V.$$
D
Electrons flow from magnesium electrode to hydrogen electrode.
Answer :
Reduction takes place at magnesium electrode and oxidation at $$SHE.$$
323. The correct order of $$E_{_{\frac{{{M^{2 + }}}}{M}}}^ \circ $$ values with negative sign for the four successive elements $$Cr, Mn, Fe$$ and $$Co$$ is
A
$$Mn > Cr > Fe > Co$$
B
$$Cr < Fe > Mn > Co$$
C
$$Fe > Mn > Cr > Co$$
D
$$Cr > Mn > Fe > Co$$
Answer :
$$Mn > Cr > Fe > Co$$
324.
Two Faraday of electricity is passed through a solution of $$CuS{O_4}.$$ The mass of copper deposited at the cathode is
$$\left( {{\text{ at}}{\text{. mass of}}\,Cu = 63.5\,amu} \right)$$
A
$$2 g$$
B
$$127 g$$
C
$$0 g$$
D
$$63.5 g$$
Answer :
$$63.5 g$$
325. $$E_{\frac{{F{e^{2 + }}}}{{Fe}}}^ \circ = - 0.441\,V$$ and $$E_{\frac{{F{e^{3 + }}}}{{F{e^{2 + }}}}}^ \circ = 0.771\,V$$ the standard $$emf$$ of the reaction $$Fe + 2F{e^{3 + }} \to 3F{e^{2 + }}$$ will be
A
0.111$$\,V$$
B
0.330$$\,V$$
C
1.653$$\,V$$
D
1.212$$\,V$$
Answer :
1.212$$\,V$$
326. Small quantities of solutions of compounds $$TX,$$ $$TY$$ and $$TZ$$ are put into separate test tubes containing $$X, Y$$ and $$Z$$ solution. $$TX$$ does not react with any of these. $$TY$$ reacts with both $$X$$ and $$Z.$$ $$TZ$$ reacts with $$X.$$ The decreasing order of state of oxidation of the anions $${X^ - },{Y^ - },{Z^ - }$$ is
A
$${Y^ - },{Z^ - },{X^ - }$$
B
$${Z^ - },{X^ - },{Y^ - }$$
C
$${Y^ - },{X^ - },{Z^ - }$$
D
$${X^ - },{Z^ - },{Y^ - }$$
Answer :
$${Y^ - },{Z^ - },{X^ - }$$
327. Conductance of $$0.1\,M\,KCl$$ ( conductivity $$ = X\,Oh{m^{ - 1}}c{m^{ - 1}})$$ filled in a conductivity cell is $$Y\,Oh{m^{ - 1}}.$$ If the conductance of $$0.1\,M\,NaOH$$ filled in the same cell is $$Z\,Oh{m^{ - 1}},$$ the molar conductance of $$NaOH$$ will be
A
$${10^3}\frac{{XZ}}{Y}$$
B
$${10^4}\frac{{XZ}}{Y}$$
C
$$10\frac{{XZ}}{Y}$$
D
$$0.1\frac{{XZ}}{Y}$$
Answer :
$${10^4}\frac{{XZ}}{Y}$$
328. The position of some metals in the electrochemical series in decreasing electropositive character is given as $$Mg > Al > Zn > Cu > Ag.$$ What will happen if a copper spoon is used to stir a solution of aluminium nitrate?
A
The spoon will get coated with aluminium.
B
An alloy of copper and aluminium is formed.
C
The solution becomes blue.
D
There is no reaction.
Answer :
There is no reaction.
329. Which of the following will form a cell with the highest voltage?
A
$$1\,M\,A{g^ + },1M\,C{o^{2 + }}$$
B
$$2\,M\,A{g^ + },2\,M\,C{o^{2 + }}$$
C
$$0.1\,M\,A{g^ + },2\,M\,C{o^{2 + }}$$
D
$$2\,M\,A{g^ + },0.1\,M\,C{o^{2 + }}$$
Answer :
$$2\,M\,A{g^ + },0.1\,M\,C{o^{2 + }}$$