111. Total volume of atoms present in a $$fcc$$ unit cell of a metal with radius $$r$$ is
A
$$\frac{{12}}{3}\pi {r^3}$$
B
$$\frac{{16}}{3}\pi {r^3}$$
C
$$\frac{{20}}{3}\pi {r^3}$$
D
$$\frac{{24}}{3}\pi {r^3}$$
Answer :
$$\frac{{16}}{3}\pi {r^3}$$
112. When molten zinc is converted into solid state, it acquires $$hcp$$ structure. The number of nearest neighbours of $$Zn$$ will be
A
6
B
12
C
8
D
4
Answer :
12
113. In acompound, atoms of element $$Y$$ form $$ccp$$ lattice and those of element $$X$$ occupy 2/3rd of tetrahedral voids. The formula of the compound will be
A
$${X_4}{Y_3}$$
B
$${X_2}{Y_3}$$
C
$${X_2}Y$$
D
$${X_3}{Y_4}$$
Answer :
$${X_4}{Y_3}$$
114. Potassium crystallizes with a
A
body-centred cubic lattice
B
face-centred cubic lattice
C
simple cubic lattice
D
orthorhombic lattice
Answer :
body-centred cubic lattice
115. Cations are present in the interstitial sites in _________.
A
Frenkel defect
B
Schottky defect
C
vacancy defect
D
metal deficiency defect
Answer :
Frenkel defect
116. Which of the following conditions favours the existence of a substance in the solid state ?
A
High temperature
B
Low temperature
C
High thermal energy
D
Weak cohesive forces
Answer :
Low temperature
117.
The arrangement of $${X^ - }$$ ions around $${A^ + }$$ ion in solid $$AX$$ is given in the figure (not drawn to scale). If the radius of $${X^ - }$$ is 250 pm, the radius of $${A^ + }$$ is
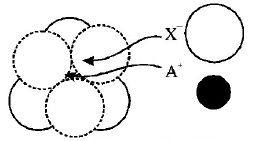
A
$$104 pm$$
B
$$183 pm$$
C
$$125 pm$$
D
$$57 pm$$
Answer :
$$104 pm$$
118. Which of the following statements is not true about the hexagonal close packing ?
A
The coordination number is 12.
B
It has 74% packing efficiency.
C
Tetrahedral voids of the second layer are covered by the spheres of the third layer.
D
In this arrangement spheres of the fourth layer are exactly aligned with those of the first layer.
Answer :
In this arrangement spheres of the fourth layer are exactly aligned with those of the first layer.
119.
A compound $${M_p}{X_q}$$ has cubic close packing $$(ccp)$$ arrangement of $$X.$$ Its unit cell structure is shown below. The empirical formula of the compound is
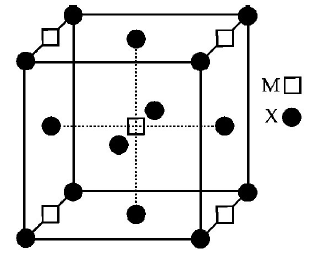
A
$$MX$$
B
$$M{X_2}$$
C
$${M_2}X$$
D
$${M_5}{X_{14}}$$
Answer :
$$M{X_2}$$
120. Compound that will show the highest lattice energy
A
$$KF$$
B
$$NaF$$
C
$$CsF$$
D
$$RbF$$
Answer :
$$NaF$$