171. The distance between $$N{a^ + }$$ and $$C{l^ - }\,ions$$ in $$NaCl$$ with a density $$3.165\,g\,c{m^{ - 3}}$$ is
A
497 $$pm$$
B
248.5 $$pm$$
C
234 $$pm$$
D
538.5 $$pm$$
Answer :
248.5 $$pm$$
172. Coordination numbers of $$C{s^ + }$$ and $$C{l^ - }$$ in $$CsCl$$ crystal are
A
8, 8
B
4, 4
C
6, 6
D
8, 4
Answer :
8, 8
173.
In the given crystal structure what should be the cation $$X$$ which replaces $$N{a^ + }$$ to create a cation vacancy ?
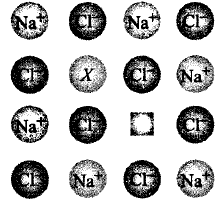
A
$$S{r^{2 + }}$$
B
$${K^ + }$$
C
$$L{i^ + }$$
D
$$B{r^ - }$$
Answer :
$$S{r^{2 + }}$$
174. An element occuring in the $$bcc$$ structure has $$12.08 \times {10^{23}}\,unit$$ cells. The total number of atoms of the element in these cells will be
A
$$24.16 \times {10^{23}}$$
B
$$36.18 \times {10^{23}}$$
C
$$6.04 \times {10^{23}}$$
D
$$12.08 \times {10^{23}}$$
Answer :
$$24.16 \times {10^{23}}$$
175. The intermetallic compound $$LiAg$$ crystallizes in a cubic lattice in which both lithium and silver atoms have coordination number of eight. To what crystal class does the unit cell belong?
A
Simple cubic
B
Face-centred cubic
C
Body-centred cubic
D
None of these
Answer :
Body-centred cubic
176. $$F{e_3}{O_4}$$ is ferrimagnetic at room temperature but at 850$$K,$$ it becomes
A
diamagnetic
B
ferromagnetic
C
paramagnetic
D
anti-ferromagnetic
Answer :
paramagnetic
177. In which of the following structures coordination number for cations and anions in the packed structure will be same ?
A
$$C{l^ - }\,ions$$ form $$fcc$$ lattice and $$N{a^ + }\,ions$$ occupy all octahedral voids of the unit cell.
B
$$C{a^{2 + }}\,ions$$ form $$fcc$$ lattice and $${F^ - }\,ions$$ occupy all the eight tetrahedral voids of the unit cell.
C
$${O^{2 - }}\,ions$$ form $$fcc$$ lattice and $$N{a^ + }\,ions$$ occupy all the eight tetrahedral voids of the unit cell.
D
$${S^{2 - }}\,ions$$ form $$fcc$$ lattice and $$Z{n^{2 + }}\,ions$$ go into alternate tetrahedral voids of the unit cell.
Answer :
$$C{l^ - }\,ions$$ form $$fcc$$ lattice and $$N{a^ + }\,ions$$ occupy all octahedral voids of the unit cell.
178. In a face centred cubic lattice, atom $$A$$ occupies the corner positions and atom $$B$$ occupies the face centre positions. If one atom of $$B$$ is missing from one of the face centred points, the formula of the compound is :
A
$${A_2}B$$
B
$$A{B_2}$$
C
$${A_2}{B_3}$$
D
$${A_2}{B_5}$$
Answer :
$${A_2}{B_5}$$
179.
In the following figure, the blank $$X$$ is known as _________ and why ?
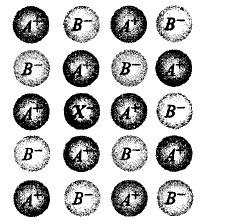
A
Electron trap, because an electron is present here.
B
Metal deficient centre, since negative charge is present here.
C
$$F$$ - centre, since it imparts colour to the crystal.
D
$$F$$ - centre, since it is responsible for positive charge on the crystal.
Answer :
$$F$$ - centre, since it imparts colour to the crystal.
180. An element crystallises into a structure which may be described by a cubic type of unit cell having one atom on each corner of the cube and two atoms on one of its diagonals. If the volume of this unit cell is $$24 \times {10^{ - 24}}c{m^3}$$ and density of element is $$7.2\,g\,c{m^{ - 3}},$$ the number of atoms present in 200 $$g$$ of element is
A
$$3.5 \times {10^{24}}$$
B
$$5.7 \times {10^{23}}$$
C
$$6.3 \times {10^{20}}$$
D
$$1 \times {10^{10}}$$
Answer :
$$3.5 \times {10^{24}}$$