81.
In $$Ca{F_2}$$ type ( fluorite structure ) $$C{a^{2 + }}\,ions$$ form $$\underline {\left( A \right)} $$ structure and $${F^ - }\,ions$$ are present in all $$\underline {\left( B \right)} $$ voids. The coordination number of $$C{a^{2 + }}$$ is $$\underline {\left( C \right)} $$ and $${F^ - }$$ is $$\underline {\left( D \right)} $$
$$\left( A \right),\left( B \right),\left( C \right)$$ and $$\left( D \right)$$ respectively are:
| A | B | C | D | |
|---|---|---|---|---|
| (a) | $$ccp$$ | octahedral | 8 | 4 |
| (b) | $$bcc$$ | tetrahedral | 4 | 8 |
| (c) | $$ccp$$ | tetrahedral | 8 | 4 |
| (d) | $$ccp$$ | octahedral | 4 | 8 |
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(c)
82.
The cubic unit cell of a metal ( molar mass $$ = 63.55g\,mo{l^{ - 1}}$$ ) has an edge length of $$362\,pm.$$ Its density is $$8.92g\,c{m^{ - 3}}.$$
The type of unit cell is
A
primitive
B
face centered
C
body centered
D
end centered
Answer :
face centered
83. Schottky defect in crystals is observed when
A
an ion leaves its normal site and occupies an interstitial site.
B
unequal number of cations and anions are missing from the lattice.
C
density of the crystal increases.
D
equal number of cations and anions are missing from the lattice.
Answer :
equal number of cations and anions are missing from the lattice.
84. An electron trapped in an anion site in a crystal is called
A
$$F$$ - centre
B
Frenkel defect
C
Schottky defect
D
interstitial defect
Answer :
$$F$$ - centre
85. A cubic solid is made up of two elements $$P$$ and $$Q.$$ Atoms of $$P$$ are present at the corners of the cube and atoms of $$Q$$ are present at body centre. What is the formula of the compound and what are coordination numbers of $$P$$ and $$Q?$$
A
$$P{Q_2},6:6$$
B
$$PQ,6:6$$
C
$${P_2}Q,6:8$$
D
$$PQ,8:8$$
Answer :
$$PQ,8:8$$
86.
Crystal defect indicated in the diagram below is
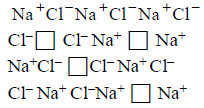
A
Interstitial defect
B
Schottky defect
C
Frenkel defect
D
Frenkel and Schottky defects
Answer :
Schottky defect
87. In the cubic close packing, the unit cell has
A
4 tetrahedral voids each of which is shared by four adjacent unit cells
B
4 tetrahedral voids within the unit cell
C
8 tetrahedral voids each of which is shared by four adjacent unit cells
D
8 tetrahedral voids within the unit cells
Answer :
8 tetrahedral voids within the unit cells
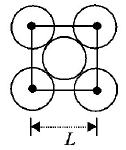
88.
The packing efficiency of the two-dimensional square unit cell shown below is :
A
39.27 %
B
68.02 %
C
74.05 %
D
78.54 %
Answer :
78.54 %
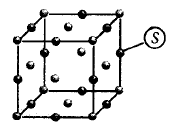
89.
For the given structure the site marked as $$S$$ is a
A
tetrahedral void
B
cubic void
C
octahedral void
D
none of these
Answer :
octahedral void
90. In a Schottky defect,
A
an ion moves to interstitial position between the lattice points
B
electrons are trapped in a lattice site
C
some lattice sites are vacant
D
some extra cations are present in interstitial spaces.
Answer :
some lattice sites are vacant