181. The van’t Hoff factor $$i$$ for a compound which undergoes dissociation in one solvent and association in other solvent is respectively :
A
less than one and greater than one.
B
less than one and less than one.
C
greater than one and less than one.
D
greater than one and greater than one.
Answer :
greater than one and less than one.
182. All form ideal solution except
A
$${C_6}{H_6}\,{\text{and}}\,{C_6}{H_5}C{H_3}$$
B
$${C_2}{H_5}Cl\,{\text{and}}\,{C_2}{H_5}l$$
C
$${C_6}{H_5}Cl\,{\text{and}}\,{C_6}{H_5}Br$$
D
$${C_2}{H_5}l\,{\text{and}}\,{C_2}{H_5}OH$$
Answer :
$${C_2}{H_5}l\,{\text{and}}\,{C_2}{H_5}OH$$
183. Which of the following is not an industrial or biological importance of osmosis ?
A
Movement of water from soil into plant roots and upper portion of plant.
B
Salting of meat to prevent bacterial action
C
Reverse osmosis for desalination of sea water.
D
Filling of ink in a fountain pen.
Answer :
Filling of ink in a fountain pen.
184. $$4\,L$$ of $$0.02\,M$$ aqueous solution of $$NaCl$$ was diluted by adding one litre of water. The molality of the resultant solution is _____________.
A
0.004$$\,m$$
B
0.008$$\,m$$
C
0.012$$\,m$$
D
0.016$$\,m$$
Answer :
0.016$$\,m$$
185. What amount of $$CaC{l_2}\left( {i = 2.47} \right)$$ is dissolved in 2 litres of water so that its osmotic pressure is $$0.5\,atm$$ at $${27^ \circ }C?$$
A
3.42$$\,g$$
B
9.24$$\,g$$
C
2.834$$\,g$$
D
1.820$$\,g$$
Answer :
1.820$$\,g$$
186. Among the following substances the lowest vapour pressure is exerted by
A
water
B
alcohol
C
ether
D
mercury.
Answer :
mercury.
187. Dissolving $$120 g$$ of urea (mol. wt. 60) in $$1000 g$$ of water gave a solution of density $$1.15 g/mL.$$ The molarity of the solution is
A
$$1.78 M$$
B
$$2.00 M$$
C
$$2.05 M$$
D
$$2.22 M$$
Answer :
$$2.05 M$$
188.
The value of Henry's law constant for some gases at $$293\,K$$ is given below. Arrange the gases in the increasing order of their solubility.
$$\eqalign{
& He:144.97\,kbar,{H_2}:69.16\,kbar, \cr
& {N_2}:76.48\,kbar,{O_2}:34.86\,kbar \cr} $$
A
$$He < {N_2} < {H_2} < {O_2}$$
B
$${O_2} < {H_2} < {N_2} < He$$
C
$${H_2} < {N_2} < {O_2} < He$$
D
$$He < {O_2} < {N_2} < {H_2}$$
Answer :
$$He < {N_2} < {H_2} < {O_2}$$
189.
In the graph plotted between vapour pressure $$(V.P.)$$ and temperature $$(T),$$
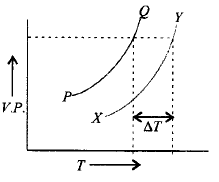
A
$$PQ$$ is the curve for solvent, $$XY$$ is the curve of solution and $$\Delta T$$ is depression in freezing point
B
$$PQ$$ is the curve for solution, $$XY$$ is the curve for solvent and $$\Delta T$$ is elevation in boiling point
C
$$PQ$$ is the curve for solvent, $$XY$$ is the curve for solution and $$\Delta T$$ is molal elevation in boiling point
D
$$PQ$$ is the curve for solvent, $$XY$$ is the curve for solution and $$\Delta T$$ is elevation in boiling point.
Answer :
$$PQ$$ is the curve for solvent, $$XY$$ is the curve for solution and $$\Delta T$$ is elevation in boiling point.
190. Choose the correct statement with respect to the vapour pressure of a liquid among the following :
A
Increases linearly with increasing temperature
B
Increases non-linearly with increasing temperature
C
Decreases linearly with increasing temperature
D
Decreases non-linearly with increasing temperature
Answer :
Increases non-linearly with increasing temperature