221. The density of a solution prepared by dissolving $$120\,g$$ of urea $$\left( {mol.{\text{ }}mass = 60{\text{ }}u} \right)$$ in $$1000\,g$$ of water is $$1.15\,g/mL.$$ The molarity of this solution is
A
1.78$$\,M$$
B
1.02$$\,M$$
C
2.05$$\,M$$
D
0.50$$\,M$$
Answer :
2.05$$\,M$$
222. The density of a solution prepared by dissolving $$120 g$$ of urea $$\left( {mol.\,mass = 60u} \right)$$ in $$1000 g$$ of water is $$1.15 g/mL.$$ The molarity of this solution is :
A
$$0.50 M$$
B
$$1.78 M$$
C
$$1.02 M$$
D
$$2.05 M$$
Answer :
$$2.05 M$$
223. A solution is made by dissolving $$20\,g$$ of a substance in $$500\,mL$$ of water. Its osmotic pressure was found to be $$600\,mm$$ of $$Hg$$ at $${15^ \circ }C.$$ Find the molecular weight of the substance.
A
1198
B
500
C
1200
D
1000
Answer :
1198
224. Determination of the molar mass of acetic acid in benzene using freezing point depression is affected by :
A
Partial ionization
B
Dissociation
C
Complex formation
D
Association
Answer :
Association
225. Which of the following aqueous solutions should have the highest osmotic pressure?
A
$$0.011M\,AlC{l_3}\,{\text{at}}\,{50^ \circ }C$$
B
$$0.03\,M\,NaCl\,{\text{at}}\,{25^ \circ }C$$
C
$$0.012\,M\left( {N{H_4}} \right)S{O_4}\,{\text{at}}\,{25^ \circ }C$$
D
$$0.03\,M\,NaCl\,{\text{at}}\,{50^ \circ }C$$
Answer :
$$0.03\,M\,NaCl\,{\text{at}}\,{50^ \circ }C$$
226. If liquids $$A$$ and $$B$$ form an ideal solution
A
the entropy of mixing is zero
B
the free energy of mixing is zero
C
the free energy as well as the entropy of mixing are each zero
D
the enthalpy of mixing is zero
Answer :
the enthalpy of mixing is zero
227. Which of the following statements is false ?
A
Two different solutions of sucrose of same molality prepared in different solvents will have the same depression in freezing point.
B
The osmotic pressure of a solution is given by the equation $$\pi = CRT$$ ( where $$C$$ is the molarity of the solution ).
C
Decreasing order of osmotic pressure for $$0.01\,M$$ aqueous solutions of barium chloride, potassium chloride, acetic acid and sucrose is $$BaC{l_2} > KCl > C{H_3}COOH > {\text{sucrose}}{\text{.}}$$
D
According to Raoult's law, the vapour pressure exerted by a volatile component of a solution is directly proportional to its mole fraction in the solution.
Answer :
Two different solutions of sucrose of same molality prepared in different solvents will have the same depression in freezing point.
228. During depression of freezing point in a solution the following are in equililbrium
A
liquid solvent, solid solvent
B
liquid solvent, solid solute
C
liquid solute, solid solute
D
liquid solute, solid solvent
Answer :
liquid solvent, solid solvent
229. For an ideal solution with $${p_A} > {p_B},$$ which of the following is true ?
A
$${\left( {{x_A}} \right)_{{\text{liquid}}}} = {\left( {{x_A}} \right)_{{\text{vapour}}}}$$
B
$${\left( {{x_A}} \right)_{{\text{liquid}}}} > {\left( {{x_A}} \right)_{{\text{vapour}}}}$$
C
$${\left( {{x_A}} \right)_{{\text{liquid}}}} < {\left( {{x_A}} \right)_{{\text{vapour}}}}$$
D
$${\left( {{x_A}} \right)_{{\text{liquid}}}}$$ and $${\left( {{x_A}} \right)_{{\text{vapour}}}}$$ do not bear any relationship with each other.
Answer :
$${\left( {{x_A}} \right)_{{\text{liquid}}}} < {\left( {{x_A}} \right)_{{\text{vapour}}}}$$
230.
Two beakers of capacity $$500\,mL$$ were taken. One of these beakers, labelled as $$''A'',$$ was filled with $$400\,mL$$ water whereas the beaker labelled $$''B''$$ was filled with $$400\,mL$$ of $$2\,M$$ solution of $$NaCl.$$ At the same temperature both the beakers were placed in closed containers of same material and same capacity as shown in figure.
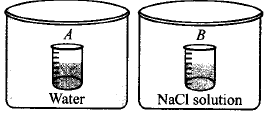
At a given temperature, which of the following statements is correct about the vapour pressure of pure water and that of $$NaCl$$ solution.
A
Vapour pressure in container $$(A)$$ is more than that in container $$(B).$$
B
Vapour pressure in container $$(A)$$ is less than that in container $$(B).$$
C
Vapour pressure is equal in both the containers.
D
Vapour pressure in container $$(B)$$ is twice the
vapour pressure in container $$(A).$$
Answer :
Vapour pressure in container $$(A)$$ is more than that in container $$(B).$$