81. On mixing $$3\,g$$ of non - volatile solute in $$200\,mL$$ of water, its boiling point $$\left( {{{100}^ \circ }} \right)$$ becomes $${100.52^ \circ }C.$$ If $${K_b}$$ for water is $$0.6\,K/m$$ then molecular $$wt.$$ of solute is :
A
$$10.5\,g\,mo{l^{ - 1}}$$
B
$$12.6\,g\,mo{l^{ - 1}}$$
C
$$15.7\,g\,mo{l^{ - 1}}$$
D
$$17.3\,g\,mo{l^{ - 1}}$$
Answer :
$$17.3\,g\,mo{l^{ - 1}}$$
82. Which of the following statement is correct if the intermolecular forces in liquids $$A, B$$ and $$C$$ are in the order $$A < B < C?$$
A
$$B$$ evaporates more readily than $$A$$
B
$$B$$ evaporates less readily than $$C$$
C
$$A$$ and $$B$$ evaporates at the same rate
D
$$A$$ evaporates more readily than $$C$$
Answer :
$$A$$ evaporates more readily than $$C$$
83. A solution of acetone in ethanol
A
shows a positive deviation from Raoult’s law
B
behaves like a non ideal solution
C
obeys Raoult’s law
D
shows a negative deviation from Raoult’s law
Answer :
shows a positive deviation from Raoult’s law
84. Which of the following liquid pairs shows a positive deviation from Raoult’s law ?
A
Water - nitric acid
B
Benzene - methanol
C
Water - hydrochloric acid
D
Acetone - chloroform
Answer :
Benzene - methanol
85.
$$1.4275\,g$$ sample of $$\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]S{O_4}Cl$$ $$\left( {mol.\,wt. = 285.5} \right)$$ is dissolved to prepare $$250\,mL$$ solution showing an osmotic pressure of $$1.478\,atm$$ at $${27^ \circ }C.$$
Which of the following statements are correct about this solution ?
(i) Each molecule furnishes three ions in solution.
(ii) The van't Hoff factor is 3.
(iii) Equilibrium molarity of $$\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]S{O_4}Cl = 0$$
(iv) Equilibrium molarity of $${\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }} = 0.02\,M$$
A
(i) and (iii) only
B
(ii) and (iv) only
C
(i), (ii) and (iv) only
D
All of these
Answer :
All of these
86. In a 0.2 molal aqueous solution of a weak acid $$HX$$ the degree of ionization is 0.3. Taking $${k_f}$$ for water as 1.85, the freezing point of the solution will be nearest to
A
$$ - {\text{ }}{0.360^ \circ }C$$
B
$$ - {\text{ }}{0.260^ \circ }C$$
C
$$ + {\text{ }}{0.480^ \circ }C$$
D
$$ - {\text{ }}{0.480^ \circ }C$$
Answer :
$$ - {\text{ }}{0.480^ \circ }C$$
87. Which one of the following statements is FALSE ?
A
The correct order of osmotic pressure for $$0.01 M$$ aqueous solution of each compound is $$BaC{l_2} > KCl > C{H_3}COOH > {\text{sucrose}}$$
B
The osmotic pressure $$\left( \pi \right)$$ of a solution is given by the $$equatio{n_\pi } = MRT,$$ where $$M$$ is the molarity of the solution
C
Raoult’s law states that the vapour pressure of a component over a solution is proportional to its mole fraction
D
Two sucrose solutions of same molality prepared in different solvents will have the same freezing point depression
Answer :
Two sucrose solutions of same molality prepared in different solvents will have the same freezing point depression
88. The freezing point of equimolal aqueous solutions will be highest for :
A
$${C_6}{H_5}N{H_3}Cl\,{\text{(aniline hydrochloride)}}$$
B
$$Ca{\left( {N{O_3}} \right)_2}$$
C
$$La{\left( {N{O_3}} \right)_3}$$
D
$${C_6}{H_{12}}{O_6}\,\,{\text{(glucose)}}$$
Answer :
$${C_6}{H_{12}}{O_6}\,\,{\text{(glucose)}}$$
89. The relative lowering of vapour pressure is equal to the ratio between the number of
A
solute molecules to the solvent molecules
B
solute molecules to the total molecules in solution
C
solvent molecules to the total molecules in the solution
D
solvent molecules to the total number of ions of the solute
Answer :
solute molecules to the total molecules in solution
90.
The given graph shows the vapour pressure temperature curves for some liquids.
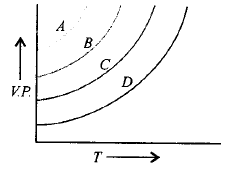
Liquids $$A, B, C$$ and $$D$$ respectively are
A
diethyl ether, acetone, ethyl alcohol, water
B
acetone, ethyl alcohol, diethyl ether, water
C
water, ethyl alcohol, acetone, diethyl ether
D
ethyl alcohol, acetone, diethyl ether, water
Answer :
diethyl ether, acetone, ethyl alcohol, water