221.
Iron can be obtained by reduction of iron oxide $$\left( {F{e_3}{O_4}} \right)$$ with $$CO$$ according to the reaction : $$F{e_3}{O_4} + 4CO \to 3Fe + 4C{O_2}$$
How many kilograms of $$F{e_3}{O_4}$$ should be heated with $$CO$$ to get $$3\,kg$$ of iron ?
A
8.12$$\,kg$$
B
4.14$$\,kg$$
C
6.94$$\,kg$$
D
16.8$$\,kg$$
Answer :
4.14$$\,kg$$
222. If Avogadro number $${N_A},$$ is changed from $$6.022 \times {10^{23}}mo{l^{ - 1}}$$ to $$6.022 \times {10^{20}}mo{l^{ - 1}}$$ this would change
A
the definition of mass in units of grams
B
the mass of one mole of carbon
C
the ratio of chemical species to each other in a balanced equation
D
the ratio of elements to each other in a compound
Answer :
the mass of one mole of carbon
223. The number of oxygen atoms in $$4.4\,g$$ of $$C{O_2}$$ is
A
$$1.2 \times {10^{23}}$$
B
$$6 \times {10^{22}}$$
C
$$6 \times {10^{23}}$$
D
$$12 \times {10^{23}}$$
Answer :
$$1.2 \times {10^{23}}$$
224. Dissolving $$120$$ $$g$$ of a compound of $$mol.$$ $$wt.$$ 60 in $$1000$$ $$g$$ of water gave a solution of density $$1.12\,g/mL.$$ The molarity of the solution is :
A
1.00$$\,M$$
B
2.00$$\,M$$
C
2.50$$\,M$$
D
4.00$$\,M$$
Answer :
2.00$$\,M$$
225. A compound contains two elements $$'X'$$ and $$'Y'$$ in the ratio of $$50\% $$ each. Atomic mass of $$'X'$$ is 20 and $$'Y'$$ is 40. What can be its simplest formula ?
A
$$XY$$
B
$${X_2}Y$$
C
$$X{Y_2}$$
D
$${X_2}{Y_3}$$
Answer :
$${X_2}Y$$
226.
Which of the following laws of chemical combinations is satisfied by the figure ?
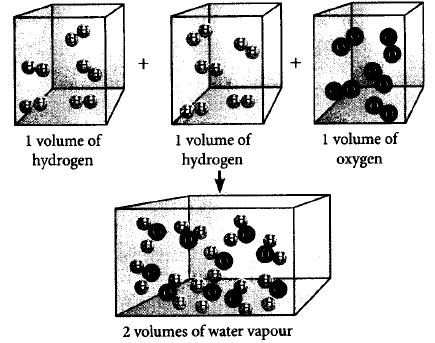
A
Law of multiple proportion
B
Law of multiple proportion
C
Gay Lussac's law of gaseous volume
D
Law of conservation of mass
Answer :
Gay Lussac's law of gaseous volume
227. How many number of aluminium ions are present in $$0.051\,g$$ of aluminium oxide ?
A
$$6.023 \times {10^{20}}\,ions$$
B
$$3\,ions$$
C
$$6.023 \times {10^{23}}\,ions$$
D
$$9\,ions$$
Answer :
$$6.023 \times {10^{20}}\,ions$$
228. Choose the incorrect formula out of the four compounds for an element $$X$$ below
A
$${X_2}{O_3}$$
B
$${X_2}C{l_3}$$
C
$${X_2}{\left( {S{O_4}} \right)_3}$$
D
$$XP{O_4}$$
Answer :
$${X_2}C{l_3}$$
229. What will be the weight of $$CO$$ having the same number of oxygen atoms as present in $$22\,g$$ of $$C{O_2}?$$
A
28$$\,g$$
B
22$$\,g$$
C
44$$\,g$$
D
72$$\,g$$
Answer :
28$$\,g$$
230. What mass of sodium chloride would be decomposed by $$9.8\,g$$ of sulphuric acid if $$12\,g$$ of sodium bisulphate and $$2.75\,g$$ of hydrogen chloride were produced in a reaction ?
A
14.75$$\,g$$
B
3.8$$\,g$$
C
4.95$$\,g$$
D
2.2$$\,g$$
Answer :
4.95$$\,g$$