201.
Following table represents critical temperature of some gases. Arrange these gases in their increasing order of liquifaction.
| Gas | $${H_2}$$ | $$He$$ | $${N_2}$$ | $${O_2}$$ |
| $${T_c}/K$$ | 33.2 | 5.3 | 126 | 154.3 |
A
$$He < {N_2} < {H_2} < {O_2}$$
B
$${H_2} < He < {N_2} < {O_2}$$
C
$$He < {H_2} < {N_2} < {O_2}$$
D
$${O_2} < {N_2} < {H_2} < He$$
Answer :
$$He < {H_2} < {N_2} < {O_2}$$
202. The vacant space in $$bcc$$ lattice cell is
A
26%
B
48%
C
23%
D
32%
Answer :
32%
203. At which one of the following temperature pressure conditions, the deviation of a gas from ideal behaviour is expected to be minimum?
A
$$350\,K\,{\text{and}}\,3\,atm$$
B
$$550\,K\,{\text{and}}\,1\,atm$$
C
$$250\,K\,{\text{and}}\,4\,atm$$
D
$$450\,K\,{\text{and}}\,2\,atm$$
Answer :
$$550\,K\,{\text{and}}\,1\,atm$$
204.
Which of the following graphs represents the correct Boyle's law ?
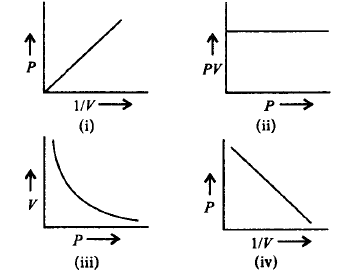
A
(i), (ii) and (iii)
B
(i) and (iv)
C
(ii) and (iii)
D
(i), (ii) and (iv)
Answer :
(i), (ii) and (iii)
205. When electrons are trapped into the crystalline anion vacancy, the defect is known as
A
Schottky defect
B
Stoichiometric defect
C
Frenkel defect
D
$$F$$ - centres
Answer :
$$F$$ - centres
206. If $$'a’$$ stands for the edge length of the cubic systems : simple cubic, body centred cubic and face centred cubic, then the ratio of radii of the spheres in these systems will be respectively,
A
$$\frac{1}{2}a:\frac{{\sqrt 3 }}{4}a:\frac{1}{{2\sqrt 2 }}a$$
B
$$\frac{1}{2}a:\sqrt 3 a:\frac{1}{{\sqrt 2 }}a$$
C
$$\frac{1}{2}a:\frac{{\sqrt 3 }}{2}a:\frac{{\sqrt 2 }}{2}a$$
D
$$1a:\sqrt 3 a:\sqrt 2 a$$
Answer :
$$\frac{1}{2}a:\frac{{\sqrt 3 }}{4}a:\frac{1}{{2\sqrt 2 }}a$$
207. Which of the following values does not represent the correct value of $$R?$$
A
$$8.314\,\,Pa\,\,{m^3}\,\,{K^{ - 1}}\,\,mo{l^{ - 1}}$$
B
$$8.314 \times {10^{ - 2}}\,bar\,\,L\,\,{K^{ - 1}}\,\,mo{l^{ - 1}}$$
C
$$0.0821\,\,J\,\,{K^{ - 1}}\,\,mo{l^{ - 1}}$$
D
$$8.314\,\,J\,\,{K^{ - 1}}\,\,mo{l^{ - 1}}$$
Answer :
$$0.0821\,\,J\,\,{K^{ - 1}}\,\,mo{l^{ - 1}}$$
208. The ability of a substance to assume in two or more crystalline structure is called
A
isomerism
B
polymorphism
C
isomorphism
D
amorphism
Answer :
polymorphism
209. The correct order of viscosity of the following liquids will be
A
Water < methyl alcohol < dimethyl ether < glycerol
B
methyl alcohol < glycerol < water < dimethyl ether
C
dimethyl ether < methyl alcohol < water < glycerol
D
glycerol < dimethyl ether < water < methyl alcohol
Answer :
dimethyl ether < methyl alcohol < water < glycerol
210. Dimethyl ether decomposes as $$C{H_3}OC{H_3}\left( g \right) \to $$ $$C{H_4}\left( g \right) + CO\left( g \right) + {H_2}\left( g \right)$$ when $$C{H_3}OC{H_3}$$ decomposes to $$20\% $$ extent under certain conditions, what is the ratio of diffusion of pure $$C{H_3}OC{H_3}$$ with methane?
A
0.59 : 1
B
1.18 : 1
C
2.36 : 1
D
1.77 : 1
Answer :
2.36 : 1