31. A copper ball of mass $$100\,gm$$ is at a temperature $$T.$$ It is dropped in a copper calorimeter of mass $$100\,gm,$$ filled with $$170\,gm$$ of water at room temperature. Subsequently, the temperature of the system is found to be $$75{\,^ \circ }C.$$ $$T$$ is given by (Given : room temperature = $$30{\,^ \circ }C$$ specific heat of copper = $$0.1\,cal/gm{\,^ \circ }C$$ )
A
$$1250{\,^ \circ }C$$
B
$$825{\,^ \circ }C$$
C
$$800{\,^ \circ }C$$
D
$$885{\,^ \circ }C$$
Answer :
$$885{\,^ \circ }C$$
32. The temperature of equal masses of three different liquids $$A,B$$ and $$C$$ are $${12^ \circ }C,{19^ \circ }C$$ and $$28^ \circ C$$ respectively. The temperature when $$A$$ and $$B$$ are mixed is $$16^ \circ C$$ and when $$B$$ and $$C$$ are mixed is $${23^ \circ }C.$$ The temperature when $$A$$ and $$C$$ are mixed is
A
$${18.2^ \circ }C$$
B
$${22^ \circ }C$$
C
$${20.2^ \circ }C$$
D
$${25.2^ \circ }C$$
Answer :
$${20.2^ \circ }C$$
33. If mass-energy equivalence is taken into account, when water is cooled to form ice, the mass of water should
A
increase
B
remain unchanged
C
decrease
D
first increase then decrease
Answer :
decrease
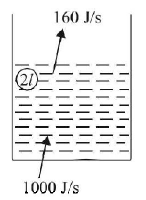
34. Water of volume 2 litre in a container is heated with a coil of $$1\,k W$$ at $$27{\,^ \circ }C.$$ The lid of the container is open and energy dissipates at rate of $$160\, J/s.$$ In how much time temperature will rise from $$27{\,^ \circ }C$$ to $$77{\,^ \circ }C$$ [Given specific heat of water is $$4.2\, kJ/kg$$ ]
A
$$7 \,min$$
B
$$6\, min\, 2\, s$$
C
$$8\, min\, 20\, s$$
D
$$14\, min$$
Answer :
$$8\, min\, 20\, s$$
35. A beaker contains $$200\,gm$$ of water. The heat capacity of the beaker is equal to that of $$20\,gm$$ of water. The initial temperature of water in the beaker is $${20^ \circ }C.$$ If $$440\,gm$$ of hot water at $${92^ \circ }C$$ is poured in it, the final temperature, neglecting radiation loss, will be nearest to
A
$${58^ \circ }C$$
B
$${68^ \circ }C$$
C
$${73^ \circ }C$$
D
$${78^ \circ }C$$
Answer :
$${68^ \circ }C$$
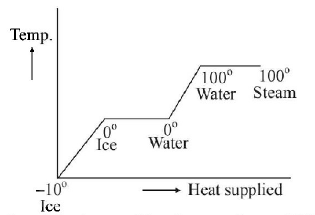
36. A block of ice at $$ - {10^ \circ }C$$ is slowly heated and converted to steam at $${100^ \circ }C.$$ Which of the following curves represents the phenomenon qualitatively?
A

B
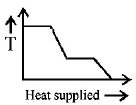

C
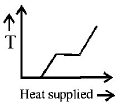

D
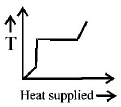

Answer :


37.
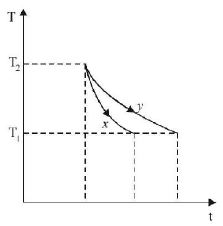
The graph, shown in the adjacent diagram, represents the variation of temperature $$(T)$$ of two bodies, $$x$$ and $$y$$ having same surface area, with time $$(t)$$ due to the emission of radiation. Find the correct relation between the emissivity and absorptivity power of the two bodies
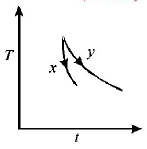
A
$${E_x} > {E_y}\,\,\& \,\,{a_x} < {a_y}$$
B
$${E_x} < {E_y}\,\,\& \,\,{a_x} > {a_y}$$
C
$${E_x} > {E_y}\,\,\& \,\,{a_x} > {a_y}$$
D
$${E_x} < {E_y}\,\,\& \,\,{a_x} < {a_y}$$
Answer :
$${E_x} > {E_y}\,\,\& \,\,{a_x} > {a_y}$$
38. An ideal Black - body at room temperature is thrown into a furnace. It is observed that
A
initially it is the darkest body and at later times the brightest
B
it is the darkest body at all times
C
it cannot be distinguished at all times
D
initially it is the darkest body and at later times it cannot be distinguished
Answer :
initially it is the darkest body and at later times the brightest
39. $$4200\,J$$ of work is required for
A
increasing the temperature of $$10\,g$$ of water through $${10^ \circ }C$$
B
increasing the temperature of $$100\,g$$ of water through $${10^ \circ }C$$
C
increasing the temperature of $$1\,kg$$ of water through $${10^ \circ }C$$
D
increasing the temperature of $$500\,g$$ of water through $${10^ \circ }C$$
Answer :
increasing the temperature of $$100\,g$$ of water through $${10^ \circ }C$$
40. Mass of water which absorbs or emits the same amount of heat as is done by the body for the same rise or fall in temperature is known as
A
thermal capacity of the body
B
specific heat capacity of the body
C
latent heat capacity of the body
D
water equivalent of the body
Answer :
water equivalent of the body