111. Formation of covalent bonds in compounds exhibits
A
wave nature of electron
B
particle nature of electron
C
both wave and particle nature of electron
D
none of these
Answer :
wave nature of electron
112. Which of the following is not the property of cathode rays?
A
It produces heating effect
B
It does not deflect in electric field
C
Its casts shadow
D
It produces fluorescence
Answer :
It does not deflect in electric field
113. The work function for sodium surface is $$2.0\,eV$$ and that for aluminium surface is $$4.2\,eV.$$ The two metal are illuminated with appropriate radiation so as to cause photo emission. Then
A
the threshold frequency for sodium will be less than that for aluminium
B
the threshold frequency for sodium will be more than that of aluminium
C
both sodium and aluminium will have the same threshold frequency
D
none of the above
Answer :
the threshold frequency for sodium will be less than that for aluminium
114. If the kinetic energy of the particle is increased to 16 times its previous value, the percentage change in the de-Broglie wavelength of the particle is
A
25
B
75
C
60
D
50
Answer :
75
115. Kinetic energy of an electron which is accelerated in a potential difference of $$100\,V$$ is
A
$$1.6 \times {10^{ - 17}}J$$
B
$$1.6 \times {10^{ - 19}}J$$
C
$$1.6 \times {10^{ - 21}}J$$
D
$$1.6 \times {10^{ - 25}}J$$
Answer :
$$1.6 \times {10^{ - 17}}J$$
116. Light of wavelength $$500\,nm$$ is incident on a metal with work function $$2.28\,eV.$$ The wavelength of the emitted electron is:
A
$$ < 2.8 \times {10^{ - 9}}m$$
B
$$ \geqslant 2.8 \times {10^{ - 9}}m$$
C
$$ \leqslant 2.8 \times {10^{ - 12}}m$$
D
$$ < 2.8 \times {10^{ - 10}}m$$
Answer :
$$ \geqslant 2.8 \times {10^{ - 9}}m$$
117. An electron beam is accelerated by a potential difference $$V$$ to hit a metallic target to produce X-rays. It produces continuous as well as characteristic X-rays.If $${\lambda _{\min }}$$ is the smallest possible wavelength of X-ray in the spectrum, the variation of $$\log {\lambda _{\min }}$$ with $$\log \,V$$ is correctly represented in :
A


B


C
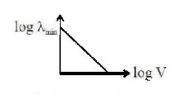

D


Answer :


118. A sensor is exposed for time $$t$$ to a lamp of power $$P$$ placed at a distance $$l.$$ The sensor has an opening that is $$4d$$ in diameter. Assuming all energy of the lamp is given off as light, the number of photons entering the sensor if the wavelength of light is $$\lambda $$ is
A
$$N = \frac{{P\lambda {d^2}t}}{{hc{l^2}}}$$
B
$$N = \frac{{4P\lambda {d^2}t}}{{hc{l^2}}}$$
C
$$N = \frac{{P\lambda {d^2}t}}{{4hc{l^2}}}$$
D
$$N = \frac{{P\lambda {d^2}t}}{{16\,hc{l^2}}}$$
Answer :
$$N = \frac{{P\lambda {d^2}t}}{{hc{l^2}}}$$
119.
An $$\alpha $$-particle having a de-Broglie wavelength $${\lambda _i}$$ collides with a stationary carbon nucleus. The $$\alpha $$-particle moves off in a different direction as shown below.

After the collision, the de Broglie wavelengths of the $$\alpha $$-particle and the carbon nucleus are $${\lambda _f}$$ and $${\lambda _e}$$ respectively. Which of the following relations about de Broglie wavelengths is correct
A
$${\lambda _i} < {\lambda _f}$$
B
$${\lambda _i} > {\lambda _f}$$
C
$${\lambda _f} = {\lambda _e}$$
D
$${\lambda _i} = {\lambda _e}$$
Answer :
$${\lambda _i} < {\lambda _f}$$
120. The shortest wavelength of X-ray emitted from an X-ray tube operated at $$2 \times {10^6}\,volt,$$ is of the order of
A
$${10^{ - 5}}\mathop {\text{A}}\limits^ \circ $$
B
$${10^{ - 2}}\mathop {\text{A}}\limits^ \circ $$
C
$$0.15\,\mathop {\text{A}}\limits^ \circ $$
D
$$1\,\mathop {\text{A}}\limits^ \circ $$
Answer :
$${10^{ - 2}}\mathop {\text{A}}\limits^ \circ $$