21. A mixture consists of two radioactive materials $${A_1}$$ and $${A_2}$$ with half lives of $$20\,s$$ and $$10\,s$$ respectively. Initially the mixture has $$40\,g$$ of $${A_1}$$ and $$160\,g$$ of $${A_2}.$$ The amount of the two in the mixture will become equal after
A
$$60\,s$$
B
$$80\,s$$
C
$$20\,s$$
D
$$40\,s$$
Answer :
$$40\,s$$
22. Beta rays emitted by a radioactive material are
A
electromagnetic radiations
B
the electrons orbiting around the nucleus
C
charged particles emitted by the nucleus
D
neutral particles
Answer :
charged particles emitted by the nucleus
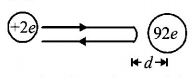
23. An alpha particle of energy $$5\,MeV$$ is scattered through $${180^ \circ }$$ by a fixed uranium nucleus. The distance of closest approach is of the order of
A
$$1\, \mathop {\text{A}}\limits^ \circ $$
B
$${10^{ - 10}}cm$$
C
$${10^{ - 12}}cm$$
D
$${10^{ - 15}}cm$$
Answer :
$${10^{ - 12}}cm$$
24. For a radioactive sample the counting rate changes from $$6520$$ counts/minute to $$3260$$ counts/minute in $$2$$ minutes. Determine the decay constant.
A
$$1.78\,{\text{per}}\,\sec $$
B
$$0.78\,{\text{per}}\,\sec $$
C
$$2.78\,{\text{per}}\,\sec $$
D
$$5.78\,{\text{per}}\,\sec $$
Answer :
$$5.78\,{\text{per}}\,\sec $$
25. Consider a radioactive material of half-life 1.0 minute. If one of the nuclei decays now, the next one will decay
A
after 1 minute
B
after $$\frac{1}{{{{\log }_e}2}}$$ minute
C
after $$\frac{1}{N}$$ minute, where $$N$$ is the number of nuclei present at that moment
D
after any time
Answer :
after any time
26. An accident in a nuclear laboratory resulted in deposition of a certain amount of radioactive material of half-life 18 days inside the laboratory. Tests revealed that the radiation was 64 times more than the permissible level required for safe operation of the laboratory. What is the minimum number of days after which the laboratory can be considered safe for use?
A
64
B
90
C
108
D
120
Answer :
108
27. In the reaction $$_1^2H + _1^3H \to _2^4He + _0^1n,$$ if the binding energies of $$_1^2H,\,_1^3H$$ and $$_2^4He$$ are respectively $$a, b$$ and $$c$$ (in $$MeV$$ ), then the energy (in $$MeV$$ ) released in this reaction is
A
$$c + a - b$$
B
$$c - a - b$$
C
$$a + b + c$$
D
$$a + b - c$$
Answer :
$$c - a - b$$
28. If nuclei of a radioactive element is produced at constant rate $$\alpha $$ and they decays with decay constant $$\lambda .$$ At $$t = 0,$$ number of nuclei is zero than the number of nuclei at time $$t$$ is
A
$$\frac{\alpha }{\lambda }\left( {1 - {e^{ - \lambda t}}} \right)$$
B
$$\alpha - \frac{\alpha }{\lambda }{e^{ - \lambda t}}$$
C
$$\frac{\alpha }{\lambda }{e^{ - \lambda t}}$$
D
$$\alpha \left( {1 - {e^{ - \lambda t}}} \right)$$
Answer :
$$\frac{\alpha }{\lambda }\left( {1 - {e^{ - \lambda t}}} \right)$$
29. In radioactive decay process, the negatively charged emitted $$\beta $$-particles are
A
the electrons present inside the nucleus
B
the electrons produced as a result of the decay of neutrons inside the nucleus
C
the electrons produced as a result of collisions between atoms
D
the electrons orbiting around the nucleus
Answer :
the electrons produced as a result of the decay of neutrons inside the nucleus
30. A radioactive sample at any instant has its disintegration rate 5000 disintegrations per minute. After 5 minutes, the rate is 1250 disintegrations per minute. Then, the decay constant (per minute) is
A
$$0.4\,\ln \,2$$
B
$$0.2\,\ln \,2$$
C
$$0.1\,\ln \,2$$
D
$$0.8\,\ln \,2$$
Answer :
$$0.4\,\ln \,2$$