171. The correct order of the $$O - O$$ bond length in $${O_2},{H_2}{O_2}$$ and $${O_3}$$ is
A
$${O_2} > {O_3} > {H_2}{O_2}$$
B
$${O_3} > {H_2}{O_2} > {O_2}$$
C
$${O_2} > {H_2}{O_2} > {O_3}$$
D
$${H_2}{O_2} > {O_3} > {O_2}$$
Answer :
$${H_2}{O_2} > {O_3} > {O_2}$$
172. The compound which contains both ionic and covalent bonds is
A
$$C{H_4}$$
B
$${H_2}$$
C
$$KCN$$
D
$$KCl$$
Answer :
$$KCN$$
173. The compound $$M{X_4}$$ is tetrahedral. The number of $$ < XMX$$ formed in the compound are
A
three
B
four
C
five
D
six
Answer :
six
174. Among the following, the pair in which the two species are not isostructural, is
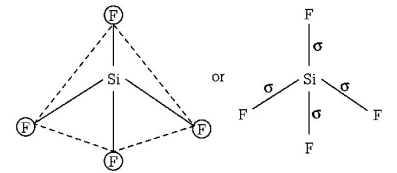
A
$$Si{F_4}\,{\text{and}}\,S{F_4}$$
B
$$lO_3^ - \,{\text{and}}\,Xe{O_3}$$
C
$$BH_4^ - \,{\text{and}}\,NH_4^ + $$
D
$$PF_6^ - \,{\text{and}}\,S{F_6}\,$$
Answer :
$$Si{F_4}\,{\text{and}}\,S{F_4}$$
175. Which of the following bond orders is indication of existence of a molecule?
A
Zero bond order
B
Negative bond order
C
Positive bond order
D
All of these
Answer :
Positive bond order
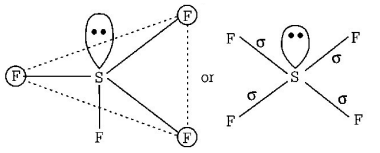
176. Which of the following shows $$ds{p^2}$$ hybridisation and a square planar geometry?
A
$$S{F_6}$$
B
$$Br{F_5}$$
C
$$PC{l_5}$$
D
$${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$$
Answer :
$${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$$
177.
Match the molecules given in column I with their shapes given in column II and mark the appropriate choice.
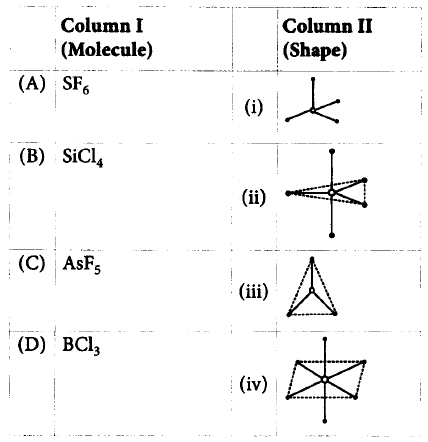
A
A - iv, B - ii, C - iii, D - i
B
A - iv, B - i, C - ii, D - iii
C
A - iii, B - i, C -ii, D - iv
D
A - ii, B - iii, C - i, D - iv
Answer :
A - iv, B - i, C - ii, D - iii
178.
The correct order of bond lengths $$P, Q$$ and $$R$$ is
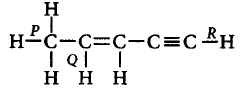
A
$$P > Q > R$$
B
$$R > Q > P$$
C
$$Q > P > R$$
D
$$Q > R > P$$
Answer :
$$P > Q > R$$
179. Given below is the bond angle in various types of hybridisation. Mark the bond angle which is not correctly matched.
A
$$ds{p^2} - {90^ \circ }$$
B
$$s{p^3}{d^2} - {90^ \circ }$$
C
$$s{p^3}d - {90^ \circ }$$
D
$$s{p^3} - {109.5^ \circ }$$
Answer :
$$s{p^3}d - {90^ \circ }$$
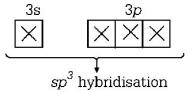
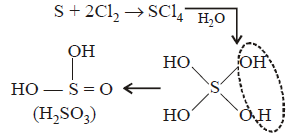
180. Sulphur reacts with chlorine in 1 : 2 ratio and forms $$X.$$ Hydrolysis of $$X$$ gives a sulphur compound $$Y.$$ What is the hybridisation state of central atom in the compound.
A
$$s{p^3}$$
B
$$s{p^2}$$
C
$$sp$$
D
$$ds{p^2}$$
Answer :
$$s{p^3}$$
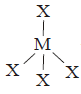 three angle below $$M$$ and three above $$M$$ hence $$= 6$$
three angle below $$M$$ and three above $$M$$ hence $$= 6$$