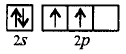
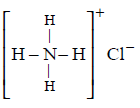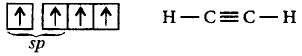181. Which one of the following is not paramagnetic?
A
$$NO$$
B
$$N_2^ + $$
C
$$CO$$
D
$$O_2^ - $$
Answer :
$$CO$$
182. Which of the following contains both covalent and ionic bond?
A
$$N{H_4}Cl$$
B
$${H_2}O$$
C
$$CC{l_4}$$
D
$$CaC{l_2}$$
Answer :
$$N{H_4}Cl$$
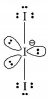
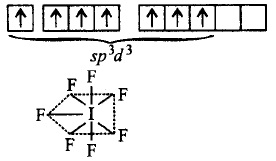
183. Total number of lone pair of electrons in \[I_3^ - \] ion is :
A
\[3\]
B
\[\,6\]
C
\[9\]
D
\[12\]
Answer :
\[9\]
184. Which of the following is paramagnetic?
A
$$CO$$
B
$$O_2^ - $$
C
$$C{N^ - }$$
D
$$N{O^ + }$$
Answer :
$$O_2^ - $$
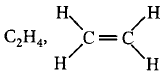
185. In which of the following molecule/ion all the bonds are not equal?
A
$$Xe{F_4}$$
B
$$BF_4^ - $$
C
$${C_2}{H_4}$$
D
$$Si{F_4}$$
Answer :
$${C_2}{H_4}$$
186. Which of the following pairs are isostructural?
A
$$SO_4^{2 - }\,{\text{and}}\,BF_4^ - $$
B
$$N{H_3}\,{\text{and}}\,NH_4^ + $$
C
$$CO_3^{2 - }\,{\text{and}}\,C{O_2}$$
D
$$C{H_4}\,{\text{and}}\,B{F_3}$$
Answer :
$$SO_4^{2 - }\,{\text{and}}\,BF_4^ - $$
187.
Match the column I with column II and mark the appropriate choice.
| Column I | Column II | ||
|---|---|---|---|
| a. | $${C_2}{H_2}$$ | 1. | $$s{p^3}{d^2}$$ hybridisation |
| b. | $$S{F_6}$$ | 2. | $$s{p^3}{d^3}$$ hybridisation |
| c. | $$S{O_2}$$ | 3. | $$sp$$ hybridisation |
| d. | $$I{F_7}$$ | 4. | $$s{p^2}$$ hybridisation |
A
a - 1, b - 3, c - 2, d - 4
B
a - 3, b - 1, c - 4, d - 2
C
a - 2, b - 3, c - 1, d - 2
D
a - 4, b - 1, c - 3, d - 2
Answer :
a - 3, b - 1, c - 4, d - 2
188. Which one of the following molecules contain no $$\pi {\text{ - bond?}}$$
A
$$C{O_2}$$
B
$${H_2}O$$
C
$$S{O_2}$$
D
$$N{O_2}$$
Answer :
$${H_2}O$$
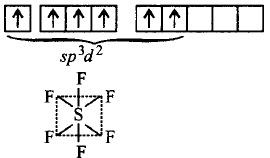
189. $$C{F_4},S{F_4}$$ and $$Xe{F_4}$$ contain the following electronic structures on their central atoms. Which one is correct option?
A
1, 2 and 3 lone pairs of electrons respectively
B
0, 1 and 2 lone pairs of electrons respectively
C
1, 1 and 1 lone pairs of electrons respectively
D
No lone pairs of electrons on any molecule
Answer :
0, 1 and 2 lone pairs of electrons respectively
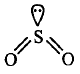
190. The correct order of bond angles (smallest first) in $${H_2}S,\,N{H_3},B{F_3}\,{\text{and}}\,Si{H_4}$$ is
A
$${H_2}S < N{H_3} < Si{H_4} < B{F_3}$$
B
$$N{H_3} < {H_2}S < Si{H_4} < B{F_3}$$
C
$${H_2}S < Si{H_4} < N{H_3} < B{F_3}$$
D
$${H_2}S < N{H_3} < B{F_3} < Si{H_4}$$
Answer :
$${H_2}S < N{H_3} < Si{H_4} < B{F_3}$$