261. Which of the following statements is not true?
A
Ionic bonds are non-directional while covalent bonds are directional.
B
Formation of $$\pi $$ - bond shortens the distance between the two concerned atoms.
C
Ionic bond is possible between similar and dissimilar atoms.
D
Linear overlapping of atomic $$p$$ - orbitals leads to a sigma bond.
Answer :
Ionic bond is possible between similar and dissimilar atoms.
262. Hydrogen bond between two atoms is formed due to
A
displacement of electrons towards more electronegative atom resulting in fractional positive charge on hydrogen
B
displacement of electrons towards hydrogen atom resulting in a polar molecule
C
formation of a bond between hydrogen atoms of one molecule and the other
D
existence of an attractive force which binds hydrogen atoms together
Answer :
displacement of electrons towards more electronegative atom resulting in fractional positive charge on hydrogen
263.
Which one of the following has the regular tetrahedral structure ?
( Atomic nos. : $$B = 5,S = 16,Ni = 28,Xe = 54$$ )
A
$$BF_4^ - $$
B
$$S{F_4}$$
C
$$Xe{F_4}$$
D
$${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$$
Answer :
$$BF_4^ - $$
264. Which of the following two are isostructural?
A
$$Xe{F_2},\,{\text{and}}\,IF_2^ - $$
B
$$N{H_3},\,{\text{and}}\,B{F_3}$$
C
$$CO_3^{2 - },\,{\text{and}}\,SO_3^{2 - }$$
D
$$PC{l_5},\,{\text{and}}\,IC{l_5}$$
Answer :
$$Xe{F_2},\,{\text{and}}\,IF_2^ - $$
265. The bond order in $$NO\,\,{\text{is}}\,\,2.5$$ while that in $$N{O^ + }\,\,{\text{is}}\,\,3$$ . Which of the following statements is true for these two species ?
A
Bond length in $$N{O^ + }\,\,{\text{is}}$$ equal to that in $$NO$$
B
Bond length in $$NO\,$$ is greater than in $$N{O^ + }$$
C
Bond length in $$N{O^ + }$$ is greater than in $$\,NO\,$$
D
Bond length is unpredictable
Answer :
Bond length in $$NO\,$$ is greater than in $$N{O^ + }$$
266. According to $$VSEPR$$ theory,
A
the shape of the molecule depends upon the bonded electron pairs
B
pair of electrons attract each other in valence shells
C
the pairs of electrons tend to occupy such positions that minimise repulsions
D
the pairs of electrons tend to occupy such positions that minimise distances from each other
Answer :
the pairs of electrons tend to occupy such positions that minimise repulsions
267. The ion that is isoelectronic with $$CO$$ is
A
$$O_2^ - $$
B
$$N_2^ + $$
C
$$O_2^ + $$
D
$$C{N^ - }$$
Answer :
$$C{N^ - }$$
268. The types of hybrid orbitals of nitrogen in $$NO_2^ + ,NO_3^ - $$ and $$NH_4^ + $$ respectively are expected to be
A
$$sp,s{p^3}\,{\text{and}}\,s{p^2}$$
B
$$sp,s{p^2}\,{\text{and}}\,s{p^3}$$
C
$$s{p^2},sp\,{\text{and}}\,s{p^3}$$
D
$$s{p^2},s{p^3}\,{\text{and}}\,sp$$
Answer :
$$sp,s{p^2}\,{\text{and}}\,s{p^3}$$
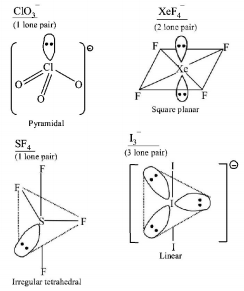
269. Which species has the maximum number of lone pair of electrons on the central atom?
A
$${\left[ {Cl{O_3}} \right]^ - }$$
B
$$Xe{F_4}$$
C
$$S{F_4}$$
D
$${\left[ {{I_3}} \right]^ - }$$
Answer :
$${\left[ {{I_3}} \right]^ - }$$
270. Which of the following represents the given mode of hybridisation $$s{p^2} - s{p^2} - sp - sp$$ from left to right ?
A
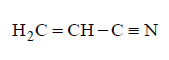
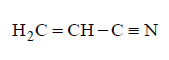
B
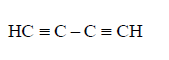
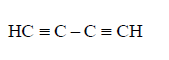
C
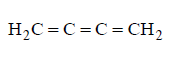
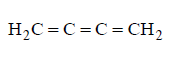
D
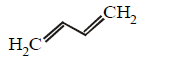

Answer :