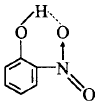
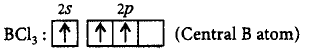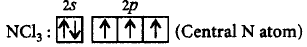321. Which of the following molecules shows intramolecular hydrogen bonding?
A
$$o$$ - Nitrophenol
B
$$p$$ - Nitrophenol
C
Benzoic acid
D
Ethanol
Answer :
$$o$$ - Nitrophenol
322. The $$BC{l_3}$$ is a planar molecule whereas $$NC{l_3}$$ is pyramidal, because
A
$$B - Cl$$ bond is more polar than $$N - Cl$$ bond
B
$$N - Cl$$ bond is more covalent than $$B - Cl$$ bond
C
nitrogen atom is smaller than boron atoms
D
$$BC{l_3}$$ has no lone pair but $$NC{l_3}$$ has a lone pair of electrons
Answer :
$$BC{l_3}$$ has no lone pair but $$NC{l_3}$$ has a lone pair of electrons
323. The formation of molecular complex $$B{F_3} - N{H_3}$$ results in a change in hybridization of boron
A
from $$s{p^2}$$ to $$ds{p^2}$$
B
from $$s{p^2}$$ to $$s{p^3}$$
C
from $$s{p^3}$$ to $$s{p^2}$$
D
from $$s{p^3}$$ to $$s{p^3}d$$
Answer :
from $$s{p^2}$$ to $$s{p^3}$$
324. In which of the following ionization processes, the bond order has increased and the magnetic behaviour has changed?
A
$${N_2} \to N_2^ + $$
B
$$\,{C_2} \to C_2^ + \,$$
C
$$NO \to N{O^ + }$$
D
$${O_2} \to O_2^ + $$
Answer :
$$NO \to N{O^ + }$$
325.
Which of the following shapes of $$S{F_4}$$ is more stable and why?
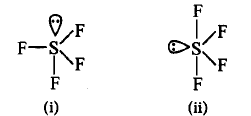
A
(i), due to $$3\,lp{\text{ - }}bp$$ repulsions at $${90^ \circ }.$$
B
(ii), due to $$2\,lp{\text{ - }}bp$$ repulsions.
C
Both are equally stable due to $$2\,lp{\text{ - }}bp$$ repulsions.
D
Both are unstable since $$S{F_4}$$ has tetrahedral shape.
Answer :
(ii), due to $$2\,lp{\text{ - }}bp$$ repulsions.
326.
Which of the following are isoelectronic and isostructural?
$$NO_3^ - ,CO_3^{2 - },ClO_3^ - ,S{O_3}$$
A
$$NO_3^ - ,CO_3^{2 - }$$
B
$$S{O_3},NO_3^ - $$
C
$$ClO_3^ - ,CO_3^{2 - }$$
D
$$CO_3^{2 - },S{O_3}$$
Answer :
$$NO_3^ - ,CO_3^{2 - }$$
327. Which of the following does not show octahedral geometry?
A
$$S{F_6}$$
B
$$I{F_5}$$
C
$$SiF_6^{2 - }$$
D
$$S{F_4}$$
Answer :
$$S{F_4}$$
328.
The electronic configuration of four atoms are given in brackets :
$$\eqalign{
& P\left( {1{s^2}2{s^2}2{p^1}} \right);Q\left( {1{s^2}2{s^2}2{p^5}} \right) \cr
& R\left( {1{s^2}2{s^2}2{p^6}3{s^1}} \right);S\left( {1{s^2}2{s^2}2{p^2}} \right) \cr} $$
The element that would most readily form a diatomic molecule is
A
$$P$$
B
$$Q$$
C
$$R$$
D
$$S$$
Answer :
$$Q$$
329. Arrange the following ions in the order of decreasing $$X – O$$ bond length, where $$X$$ is the central atom
A
$$ClO_4^ - ,SO_4^{2 - },PO_4^{3 - },SiO_4^ - $$
B
$$SiO_4^{4 - },PO_4^{3 - },SO_4^{2 - },ClO_4^ - $$
C
$$SiO_4^{4 - },PO_4^{3 - },ClO_4^ - ,SO_4^{2 - }$$
D
$$SiO_4^{4 - },SO_4^{2 - },PO_4^{3 - },ClO_4^ - $$
Answer :
$$SiO_4^{4 - },PO_4^{3 - },SO_4^{2 - },ClO_4^ - $$
330. Which one of the following pairs of molecules will have permanent dipole moments for both members ?
A
$$N{O_2}\,{\text{and}}\,C{O_2}$$
B
$$N{O_2}\,{\text{and}}\,{O_3}$$
C
$$Si{F_4}\,{\text{and}}\,C{O_2}$$
D
$$Si{F_4}\,{\text{and}}\,N{O_2}$$
Answer :
$$N{O_2}\,{\text{and}}\,{O_3}$$