341. In which of the following molecules the central atom does not retain any lone pair of electrons?
A
$$N{O_2}$$
B
$$N{H_3}$$
C
$$B{F_3}$$
D
$${H_2}O$$
Answer :
$$B{F_3}$$
342. In $$PO_4^{3 - },$$ the formal charge on each oxygen atom and the $$P - O$$ bond order respectively are
A
$$ - 0.75, 0.6$$
B
$$- 0.75, 1.0$$
C
$$ - 0.75, 1.25$$
D
$$ - 3, 1.25$$
Answer :
$$ - 0.75, 1.25$$
343. The canonical or resonating structures of a molecule required to describe the structure of a molecule follow which of the following rules?
A
The relative position of all atoms can differ.
B
The same number of unpaired and paired electrons in all structures.
C
The energy of each structure is different.
D
Like charges are present on adjacent atoms.
Answer :
The same number of unpaired and paired electrons in all structures.
344. Lattice energy of an ionic compound depends upon
A
Charge on the ion and size of the ion
B
Packing of ions only
C
Size of the ion only
D
Charge on the ion only
Answer :
Charge on the ion and size of the ion
345. Which of the following is least likely to behave as Lewis base?
A
$$N{H_3}$$
B
$$B{F_3}$$
C
$$O{H^ - }$$
D
$${H_2}O$$
Answer :
$$B{F_3}$$
346. What is the order of stability of $${N_2}$$ and its ions?
A
$${N_2} > N_2^ + = N_2^ - > N_2^{2 - }$$
B
$$N_2^ + > N_2^ - > {N_2} > N_2^{2 - }$$
C
$$N_2^ - > N_2^ + > {N_2} > N_2^{2 - }$$
D
$$N_2^{2 - } > N_2^ - = N_2^ + > {N_2}$$
Answer :
$${N_2} > N_2^ + = N_2^ - > N_2^{2 - }$$
347. Among the following, the paramagnetic compound is
A
$$N{a_2}{O_2}$$
B
$$\,{O_3}$$
C
$${N_2}O$$
D
$$K{O_2}$$
Answer :
$$K{O_2}$$
348. In $$Br{F_3}$$ molecule, the lone pairs occupy equatorial positions to minimise
A
lone pair-bond pair repulsion
B
bond pair-bond pair repulsion
C
lone pair-lone pair repulsion and lone pair-bond pair repulsion
D
lone pair-lone pair repulsion
Answer :
lone pair-lone pair repulsion
349. The number of unpaired electrons in a paramagnetic diatomic molecule of an element with atomic number 16 is
A
2
B
3
C
4
D
1
Answer :
2
350. The molecules $$B{F_3}$$ and $$N{F_3}$$ are both covalent compounds, but $$B{F_3}$$ is non polar whereas $$N{F_3}$$ is polar. The reason for this is
A
atomic size of boron is larger than nitrogen
B
Boron is metal while nitrogen is gas
C
$$B - F$$ bonds are non-polar while $$N - F$$ bonds are polar
D
$$B{F_3}$$ is planar but $$N{F_3}$$ is pyramidal
Answer :
$$B{F_3}$$ is planar but $$N{F_3}$$ is pyramidal
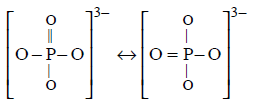
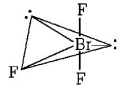
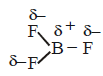 and $$\mu = 0$$ hence it is non polar.
and $$\mu = 0$$ hence it is non polar. 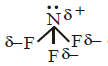 and $$\mu \ne 0$$ hence it is polar.
and $$\mu \ne 0$$ hence it is polar.