221. Type of isomerism which exists between $$\left[ {Pd{{\left( {{C_6}{H_5}} \right)}_2}{{\left( {SCN} \right)}_2}} \right]$$ and $$\left[ {Pd{{\left( {{C_6}{H_5}} \right)}_2}{{\left( {NCS} \right)}_2}} \right]$$ is :
A
Linkage isomerism
B
Coordination isomerism
C
Ionisation isomerism
D
Solvate isomerism
Answer :
Linkage isomerism
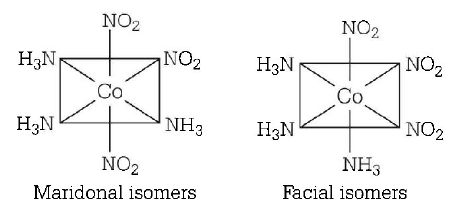
222. The number of geometrical isomers of the complex $$\left[ {Co{{\left( {N{O_2}} \right)}_3}{{\left( {N{H_3}} \right)}_3}} \right]$$ is
A
4
B
0
C
2
D
3
Answer :
2
223. Which of the following does not show optical isomerism? ( $$en =$$ ethylenediamine )
A
$${\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }$$
B
$${\left[ {Co{{\left( {N{H_3}} \right)}_3}C{l_3}} \right]^0}$$
C
$${\left[ {Co\left( {en} \right)C{l_2}{{\left( {N{H_3}} \right)}_2}} \right]^ + }$$
D
$${\left[ {Co{{\left( {en} \right)}_3}} \right]^{3 + }}$$
Answer :
$${\left[ {Co{{\left( {N{H_3}} \right)}_3}C{l_3}} \right]^0}$$
224. In which of the following octahedral complex species the magnitude of $${\Delta _ \circ }$$ will be maximum ?
A
$${\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
B
$${\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
C
$${\left[ {Co{{\left( {{C_2}{O_4}} \right)}_3}} \right]^{3 - }}$$
D
$${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
Answer :
$${\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
225.
Which of the following complex ions is expected to absorb visible light ?
$$\left( {{\text{At}}{\text{. no}}{\text{. of}}\,\,Zn = 30,Sc = 21,} \right.$$ $$\left. {Ti = 22,Cr = 24} \right)$$
A
$${\left[ {Sc{{\left( {{H_2}O} \right)}_3}{{\left( {N{H_3}} \right)}_3}} \right]^{3 + }}$$
B
$${\left[ {Ti{{\left( {en} \right)}_2}{{\left( {N{H_3}} \right)}_2}} \right]^{4 + }}$$
C
$${\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
D
$${\left[ {Zn{{\left( {N{H_3}} \right)}_6}} \right]^{2 + }}$$
Answer :
$${\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
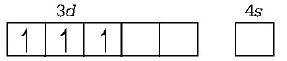
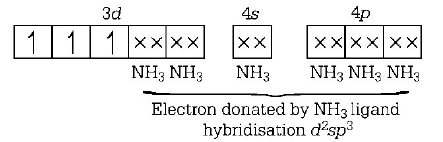
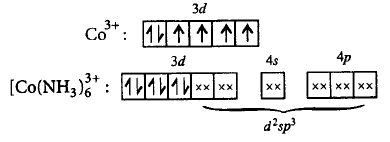
226. The hybridisation involved in $${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$ is
A
$$s{p^3}{d^2}$$
B
$$s{p^3}{d^3}$$
C
$$ds{p^3}$$
D
$${d^2}s{p^3}$$
Answer :
$${d^2}s{p^3}$$
227. The coordination number and oxidation state of $$Cr$$ in $${K_3}\left[ {Cr{{\left( {{C_2}{O_4}} \right)}_3}} \right]$$ are respectively
A
3 and +3
B
3 and 0
C
6 and +3
D
4 and +2
Answer :
6 and +3
228. The type of isomerism present in nitropentammine chromium (III) chloride is
A
optical
B
linkage
C
ionization
D
polymerisation.
Answer :
linkage
229. Which among the following will be named as dibromidobis (ethylene diamine) chromium (III) bromide?
A
$$\left[ {Cr{{\left( {en} \right)}_3}} \right]B{r_3}$$
B
$$\left[ {Cr{{\left( {en} \right)}_2}B{r_2}} \right]Br$$
C
$${\left[ {Cr\left( {en} \right)B{r_4}} \right]^ - }$$
D
$$\left[ {Cr\left( {en} \right)B{r_2}} \right]Br$$
Answer :
$$\left[ {Cr{{\left( {en} \right)}_2}B{r_2}} \right]Br$$
230. If excess of $$AgN{O_3}$$ solution is added to $$100\,mL$$ of a $$0.024\,M$$ solution of dichloro$$bis$$ (ethylenediamine) cobalt(III) chloride, how many moles of $$AgCl$$ be precipitated?
A
0.0012
B
0.0016
C
0.0024
D
0.0048
Answer :
0.0024