251. The number of unpaired electrons in the complex ion $${\left[ {Co{F_6}} \right]^{3 - }}$$ is ( At. no. of $$Co = 27$$ )
A
3
B
2
C
4
D
0
Answer :
4
252. When one mole of each of the following complexes is treated with excess of $$AgN{O_3},$$ which will give maximum amount of $$AgCl?$$
A
$$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]C{l_3}$$
B
$$\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]C{l_2}$$
C
$$\left[ {Co{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]Cl$$
D
$$\left[ {Co{{\left( {N{H_3}} \right)}_3}C{l_3}} \right]$$
Answer :
$$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]C{l_3}$$
253.
In the separation of $$C{u^{2 + }}$$ and $$C{d^{2 + }}$$ of IInd group in qualitative analysis of cations, tetrammine copper (II) sulphate and tetrammine cadmium (II) sulphate react with $$KCN$$ to form the corresponding cyano complexes, which one of the following pairs of the complexes and their relative stability enables the separation of $$C{u^{2 + }}$$ and $$C{d^{2 + }}?$$
A
$${K_3}\left[ {Cu{{\left( {CN} \right)}_4}} \right]:$$ less stable and $${K_2}\left[ {Cd{{\left( {CN} \right)}_4}} \right]:$$ more stable
B
$${K_3}\left[ {Cu{{\left( {CN} \right)}_4}} \right]:$$ more stable and $${K_2}\left[ {Cd{{\left( {CN} \right)}_4}} \right]:$$ less stable
C
$${K_2}\left[ {Cu{{\left( {CN} \right)}_4}} \right]:$$ less stable and $${K_2}\left[ {Cd{{\left( {CN} \right)}_4}} \right]:$$ more stable
D
$${K_2}\left[ {Cu{{\left( {CN} \right)}_4}} \right]:$$ more stable and $${K_2}\left[ {Cd{{\left( {CN} \right)}_4}} \right]:$$ less stable
Answer :
$${K_3}\left[ {Cu{{\left( {CN} \right)}_4}} \right]:$$ more stable and $${K_2}\left[ {Cd{{\left( {CN} \right)}_4}} \right]:$$ less stable
254. The value of the 'spin only' magnetic moment for one of the following configurations is 2.84 $$BM.$$ The correct one is
A
$${d^4}$$ ( in strong ligand field )
B
$${d^4}$$ ( in weak ligand field )
C
$${d^3}$$ ( in weak as well as in strong fields )
D
$${d^5}$$ ( in strong ligand field )
Answer :
$${d^4}$$ ( in strong ligand field )
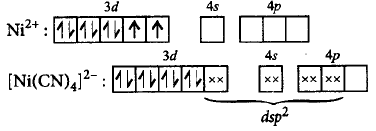
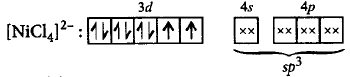
255. Give reason for the statement, $${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$$ is diamagnetic while $${\left[ {NiC{l_4}} \right]^{2 - }}$$ is paramagnetic in nature.
A
In $${\left[ {NiC{l_4}} \right]^{2 - }},$$ no unpaired electrons are present while in $${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$$ two unpaired electrons are present.
B
In $${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }},$$ no unpaired electrons are present while in $${\left[ {NiC{l_4}} \right]^{2 - }}$$ two unpaired electrons are present.
C
$${\left[ {NiC{l_4}} \right]^{2 - }}$$ shows $$ds{p^2}$$ hybridisation hence it is paramagnetic.
D
$${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$$ shows $$s{p^3}$$ hybridisation hence it is diamagnetic.
Answer :
In $${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }},$$ no unpaired electrons are present while in $${\left[ {NiC{l_4}} \right]^{2 - }}$$ two unpaired electrons are present.
256. $$CrC{l_3} \cdot 6{H_2}O$$ exists in different isomeric forms which show different colours like violet and green. This is due to
A
ionisation isomerism
B
coordination isomerism
C
optical isomerism
D
hydrate isomerism
Answer :
hydrate isomerism
257. In solid $$CuS{O_4}.5{H_2}O$$ copper is coordinated to
A
4 water molecules
B
5 water molecules
C
one sulphate molecule
D
one water molecule
Answer :
4 water molecules
258. What is the magnetic moment (spin only) and hybridisation of the brown ring complex $$\left[ {Fe{{\left( {{H_2}O} \right)}_5}NO} \right]S{O_4}?$$
A
$$\sqrt 3 BM,s{p^3}{d^2}$$
B
$$\sqrt 3 BM,{d^2}s{p^3}$$
C
$$\sqrt {15} BM,s{p^3}{d^2}$$
D
$$\sqrt {15} BM,{d^2}s{p^3}$$
Answer :
$$\sqrt {15} BM,s{p^3}{d^2}$$
259. When $$1\,mol\,CrC{l_3} \cdot 6{H_2}O$$ is treated with excess of $$AgN{O_3},3\,mol$$ of $$AgCl$$ are obtained. The formula of the complex is
A
$$\left[ {CrC{l_3}{{\left( {{H_2}O} \right)}_3}} \right] \cdot 3{H_2}O$$
B
$$\left[ {CrC{l_2}{{\left( {{H_2}O} \right)}_4}} \right]Cl \cdot 2{H_2}O$$
C
$$\left[ {CrCl{{\left( {{H_2}O} \right)}_5}} \right]C{l_2} \cdot {H_2}O$$
D
$$\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]C{l_3}$$
Answer :
$$\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]C{l_3}$$
260. The magnetic moment of the complex anion $${\left[ {Cr\left( {NO} \right)\left( {N{H_3}} \right){{\left( {CN} \right)}_4}} \right]^{2 - }}$$ is :
A
5.91$$\,BM$$
B
3.87$$\,BM$$
C
1.73$$\,BM$$
D
2.82$$\,BM$$
Answer :
2.82$$\,BM$$