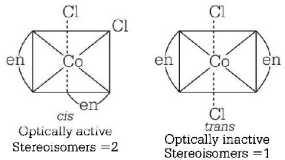
261. Which one of the following has largest number of isomers? ( $$R =$$ alkyl group, $$en =$$ ethylenediamine )
A
$${\left[ {Ru{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]^ + }$$
B
$${\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]^{2 + }}$$
C
$$\left[ {Ni{{\left( {N{H_3}} \right)}_2}C{l_2}} \right]$$
D
$${\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }$$
Answer :
$${\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }$$
262.
Match the column I with the column II and mark the appropriate choice.
| Column I | Column II | ||
|---|---|---|---|
| a. | Analytical Chemistry | 1. | $$EDTA$$ |
| b. | Volumetric estimation | 2. | Silver complexes |
| c. | Catalyst | 3. | $$C{u^{2 + }},F{e^{3 + }},N{i^{2 + }}$$ |
| d. | Electroplating | 4. | $${\left( {P{h_3}P} \right)_3}RhCl$$ |
A
a - 2, b - 3, c - 1, d - 4
B
a - 1, b - 3, c - 2, d - 4
C
a - 3, b - 1, c - 4, d - 2
D
a - 1, b - 4, c - 2, d - 3
Answer :
a - 3, b - 1, c - 4, d - 2
263.
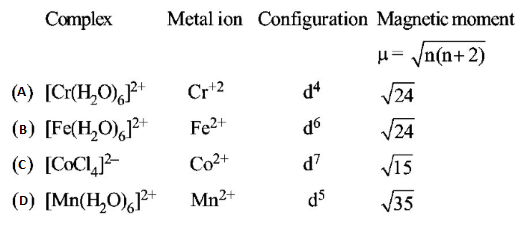
The pair having the same magnetic moment is:
$$\left[ {{\text{At}}\,{\text{No}}.:\,Cr = 24,Mn = 25,Fe = 26,Co = 27} \right]$$
A
$${\left[ {Mn{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}\,{\text{and}}\,{\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
B
$${\left[ {CoC{l_4}} \right]^{2 - }}\,{\text{and}}\,{\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
C
$${\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}\,{\text{and}}\,{\left[ {CoC{l_4}} \right]^{2 - }}$$
D
$${\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}\,{\text{and}}\,{\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
Answer :
$${\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}\,{\text{and}}\,{\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
264.
Among the following complexes $$\left( {K - P} \right):$$
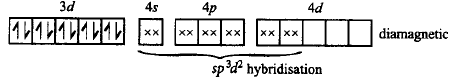
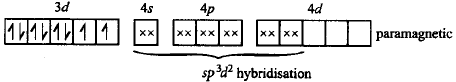
$${K_3}\left[ {Fe{{\left( {CN} \right)}_6}} \right] - K;$$ $$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]C{l_3} - L;$$ $$N{a_3}\left[ {Co{{\left( {{\text{oxalate}}} \right)}_3}} \right] - M;$$ $$\left[ {Ni{{\left( {{H_2}O} \right)}_6}} \right]C{l_2} - N;$$ $${K_2}\left[ {Pt{{\left( {CN} \right)}_4}} \right] - O$$ and $$\left[ {Zn{{\left( {{H_2}O} \right)}_6}} \right]{\left( {N{O_3}} \right)_2} - P;$$ the diamagnetic complexes are
A
$$K, L, M, N$$
B
$$K, M, O, P$$
C
$$L, M, O, P$$
D
$$L, M, N, O$$
Answer :
$$L, M, O, P$$
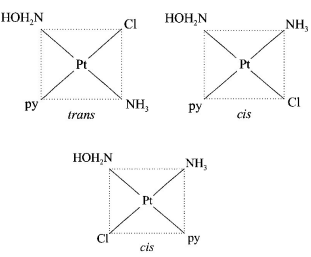
265. The number of geometric isomers that can exist for square planar complex $${\left[ {Pt\left( {Cl} \right)\left( {py} \right)\left( {N{H_3}} \right)\left( {N{H_2}OH} \right)} \right]^ + }$$ is $$\left( {py = {\text{ pyridine}}} \right):$$
A
4
B
6
C
2
D
3
Answer :
3
266. A substance appears coloured because
A
it absorbs light at specific wavelength in the visible part and reflects rest of the wavelengths
B
ligands absorb different wavelengths of light which give colour to the complex
C
it absorbs white light and shows different colours at different wavelength
D
it is diamagnetic in nature
Answer :
it absorbs light at specific wavelength in the visible part and reflects rest of the wavelengths
267.
Number of possible isomers for the complex $$\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]Cl$$ will be
( $$en=$$ ethylenediamine )
A
2
B
1
C
3
D
4
Answer :
3
268. The complex ion which has no $$'d'$$ electron in the central metal atom is
A
$${\left[ {Mn{O_4}} \right]^ - }$$
B
$${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
C
$${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
D
$${\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$$
Answer :
$${\left[ {Mn{O_4}} \right]^ - }$$
269. Which of the following has highest paramagnetism?
A
$${\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$$
B
$${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
C
$${\left[ {Cu{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
D
$${\left[ {Zn{{\left( {{H_2}O} \right)}_2}} \right]^{2 + }}$$
Answer :
$${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
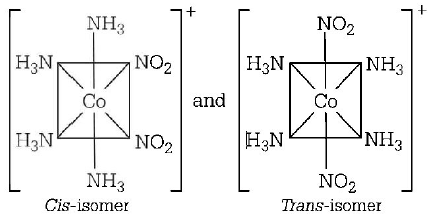
270. $$\left[ {Co{{\left( {N{H_3}} \right)}_4}{{\left( {N{O_2}} \right)}_2}} \right]Cl$$ exhibits
A
linkage isomerism, geometrical isomerism and optical isomerism
B
linkage isomerism, ionisation isomerism and optical isomerism
C
linkage isomerism, ionisation isomerism and geometrical isomerism
D
ionisation isomerism, geometrical isomerism and optical isomerism
Answer :
linkage isomerism, ionisation isomerism and geometrical isomerism