271. Which of the following compounds exhibits linkage isomerism?
A
$$\left[ {Co{{\left( {en} \right)}_3}} \right]C{l_3}$$
B
$$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]\left[ {Cr{{\left( {en} \right)}_3}} \right]$$
C
$$\left[ {Co{{\left( {en} \right)}_2}\left( {N{O_2}} \right)Cl} \right]Br$$
D
$$\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]B{r_2}$$
Answer :
$$\left[ {Co{{\left( {en} \right)}_2}\left( {N{O_2}} \right)Cl} \right]Br$$
272. Which of the following will give maximum number of isomers ?
A
$$\left[ {Co{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]$$
B
$${\left[ {Ni\left( {en} \right){{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}$$
C
$$\left[ {Ni\left( {{C_2}{O_4}} \right){{\left( {en} \right)}_2}} \right]$$
D
$${\left[ {Cr{{\left( {SCN} \right)}_2}{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}$$
Answer :
$${\left[ {Cr{{\left( {SCN} \right)}_2}{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}$$
273. Atomic number of $$Cr$$ and $$Fe$$ are respectively 24 and 26, which of the following is paramagnetic with the spin of electron ?
A
$$\left[ {Cr{{\left( {CO} \right)}_6}} \right]$$
B
$$\left[ {Fe{{\left( {CO} \right)}_5}} \right]$$
C
$${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}$$
D
$${\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
Answer :
$${\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
274. The correct structure of ethylenediaminetetraacetic acid (EDTA) is
A
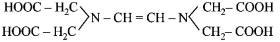
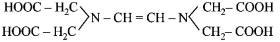
B
.PNG)
.PNG)
C
.PNG)
.PNG)
D
.PNG)
.PNG)
Answer :
.PNG)
.PNG)
275.
What are the correct oxidation state, coordination number, configuration, magnetic character and magnetic moment of $${K_4}\left[ {Mn{{\left( {CN} \right)}_6}} \right]?$$
| O.S. | C.N. | Configuration | Magnetic Character | Magnetic Moment | |
|---|---|---|---|---|---|
| (a) | +6 | 6 | $$t_{2g}^5$$ | Diamagnetic | 0 |
| (b) | +4 | 6 | $$t_{2g}^4\,e_g^1$$ | Paramagnetic | 1.732 $$B.M.$$ |
| (c) | +2 | 6 | $$t_{2g}^5$$ | Paramagnetic | 1.732 $$B.M.$$ |
| (d) | +4 | 6 | $$t_{2g}^3\,e_g^2$$ | Diamagnetic | 0 |
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(c)
276. The complex showing a spin-only magnetic moment of $$2.82\,B.M.$$ is :
A
$$Ni{\left( {CO} \right)_4}$$
B
$${\left[ {NiC{l_4}} \right]^{2 - }}$$
C
$$Ni{\left( {PP{h_3}} \right)_4}$$
D
$${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$$
Answer :
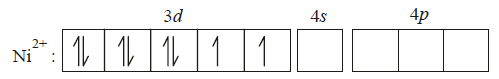
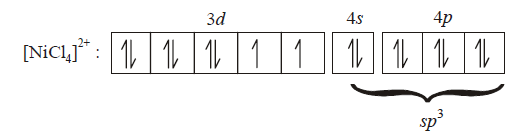
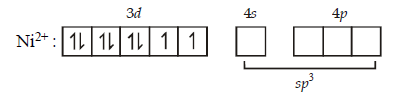
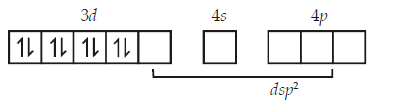
$${\left[ {NiC{l_4}} \right]^{2 - }}$$
277. $$CuS{O_4}$$ ecolourises on addition of $$KCN,$$ the product formed is
A
$$C{u^{2 + }}\,{\text{get}}\,{\text{reduced}}\,{\text{to}}\,{\text{form}}\,{\left[ {Cu{{\left( {CN} \right)}_4}} \right]^{3 - }}$$
B
$${\left[ {Cu{{\left( {CN} \right)}_4}} \right]^{2 - }}$$
C
$$CuCN$$
D
$$Cu{\left( {CN} \right)_2}$$
Answer :
$$C{u^{2 + }}\,{\text{get}}\,{\text{reduced}}\,{\text{to}}\,{\text{form}}\,{\left[ {Cu{{\left( {CN} \right)}_4}} \right]^{3 - }}$$
278. The ligand $$N{\left( {C{H_2}C{H_2}N{H_2}} \right)_3}$$ is
A
bidentate
B
tridentate
C
tridentate
D
pentadentate.
Answer :
tridentate
279. $$\left[ {NiC{l_2}{{\left\{ {P{{\left( {{C_2}{H_5}} \right)}_2}\left( {{C_6}{H_5}} \right)} \right\}}_2}} \right]$$ exhibits temperature dependent magnetic behaviour ( paramagnetic/ diamagnetic ). The coordination geometries of $$N{i^{2 + }}$$ in the paramagnetic and diamagnetic states are respectively
A
tetrahedral and tetrahedral
B
square planar and square planar
C
tetrahedral and square planar
D
square planar and tetrahedral
Answer :
tetrahedral and square planar
280. Cobalt (III) chloride forms several octahedral complexes with ammonia. Which of the following will not give test for chloride ions with silver nitrate at $${25^ \circ }C?$$
A
$$CoC{l_3} \cdot 3N{H_3}$$
B
$$CoC{l_3} \cdot 4N{H_3}$$
C
$$CoC{l_3} \cdot 5N{H_3}$$
D
$$CoC{l_3} \cdot 6N{H_3}$$
Answer :
$$CoC{l_3} \cdot 3N{H_3}$$
.PNG)