281. For which value of the $$x,$$ and $$y,$$ the following square planar compound shows geometrical isomers $${\left[ {Pt{{\left( {Cl} \right)}_x}{{\left( {Br} \right)}_y}} \right]^{2 - }}$$
A
1, 3
B
3, 1
C
2, 2
D
1, 1
Answer :
3, 1
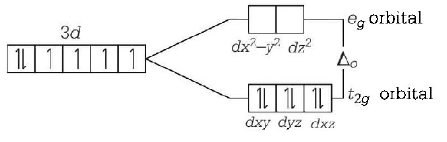
282. Among the following complexes, the one which shows zero crystal field stabilisation energy $$(CFSE)$$ is
A
$${\left[ {Mn{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$$
B
$${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$$
C
$${\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
D
$${\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$$
Answer :
$${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$$
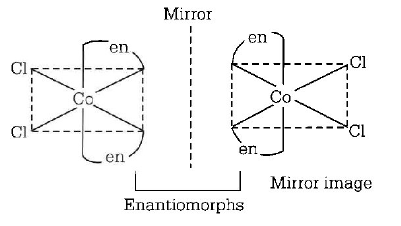
283. Which of the following will give a pair of enantiomers ? $$\left( {en = N{H_2}C{H_2}C{H_2}N{H_2}} \right)$$
A
$$\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]\left[ {Co{{\left( {CN} \right)}_6}} \right]$$
B
$$\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]Cl$$
C
$$\left[ {Pt{{\left( {N{H_3}} \right)}_4}} \right]\left[ {PtC{l_6}} \right]$$
D
$$\left[ {Co{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]N{O_2}$$
Answer :
$$\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]Cl$$
284. In nitroprusside ion, the iron and $$NO$$ exist as $$Fe\left( {{\text{II}}} \right)$$ and $$\mathop N\limits^ + O$$ rather than $$Fe\left( {{\text{III}}} \right)$$ and $$NO.$$ This can be established by
A
estimating the concentration of iron
B
estimating the concentration of $$C{N^ - }$$
C
thermally decomposing the compound
D
measuring the solid state magnetic moment
Answer :
measuring the solid state magnetic moment
285. Which of these statements about $${\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$ is true ?
A
$${\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$ has no unpaired electrons and will be in a low-spin configuration.
B
$${\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$ has four unpaired electrons and will be in a low-spin configuration.
C
$${\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$ has four unpaired electrons and will be in a high-spin configuration.
D
$${\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$ has no unpaired electrons and will
be in a high-spin configuration.
Answer :
$${\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$ has no unpaired electrons and will be in a low-spin configuration.
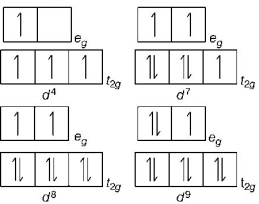
286. Jahn-Teller effect is not observed in high spin complexes of
A
$${d^7}$$
B
$${d^8}$$
C
$${d^4}$$
D
$${d^9}$$
Answer :
$${d^8}$$
287. Which of the characteristic is not common between $${\left[ {Cu{{\left( {en} \right)}_2}} \right]^{2 + }}$$ and $$\left[ {Ni{{\left( {dmg} \right)}_2}} \right]?$$
A
Geometry of complexes
B
Hybridization of central metal cation
C
Magnetic behaviour
D
Number of stereoisomers
Answer :
Magnetic behaviour
288. In the complexes $${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }},{\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{3 - }},{\left[ {Fe{{\left( {{C_2}{O_4}} \right)}_3}} \right]^{3 - }}$$ and $${\left[ {FeC{l_6}} \right]^{3 - }},$$ more stability is shown by
A
$${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$$
B
$${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
C
$${\left[ {Fe{{\left( {{C_2}{O_4}} \right)}_3}} \right]^{3 - }}$$
D
$${\left[ {FeC{l_6}} \right]^{3 - }}$$
Answer :
$${\left[ {Fe{{\left( {{C_2}{O_4}} \right)}_3}} \right]^{3 - }}$$
289. Correct formula of tetraamminechloridonitroplatinum(IV) sulphate can be written as
A
$$\left[ {Pt{{\left( {N{H_3}} \right)}_4}\left( {ONO} \right)Cl} \right]S{O_4}$$
B
$${\left[ {Pt{{\left( {N{H_3}} \right)}_4}C{l_2}N{O_2}} \right]_2}S{O_4}$$
C
$$\left[ {Pt{{\left( {N{H_3}} \right)}_4}\left( {N{O_2}} \right)Cl} \right]S{O_4}$$
D
$$\left[ {PtCl\left( {ONO} \right)N{H_3}\left( {S{O_4}} \right)} \right]$$
Answer :
$$\left[ {Pt{{\left( {N{H_3}} \right)}_4}\left( {N{O_2}} \right)Cl} \right]S{O_4}$$
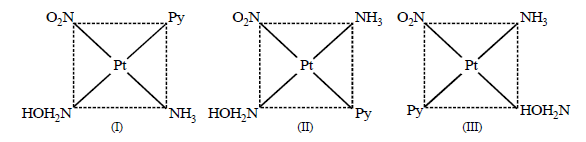
290. The complex $$ion{\left[ {Pt\left( {N{O_2}} \right)\left( {Py} \right)\left( {N{H_3}} \right)\left( {N{H_2}OH} \right)} \right]^ + }$$ will give
A
2 isomers (Geometrical)
B
3 isomers (Geometrical)
C
6 isomers (Geometrical)
D
4 isomers (Geometrical)
Answer :
3 isomers (Geometrical)