341. A solution containing $$2.675\,g$$ of $$CoC{l_3}.6N{H_3}$$ $$\left( {{\text{molar mass}} = 267.5\,g\,mo{l^{ - 1}}} \right)$$ is passed through a cation exchanger. The chloride ions obtained in solution were treated with excess of $$AgN{O_3}$$ to give $$4.78\,g$$ of $$AgCl$$ $$\left( {{\text{molar mass}} = 143.5\,g\,mo{l^{ - 1}}} \right).$$ The formula of the complex is
A
$$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]C{l_3}$$
B
$$\left[ {CoC{l_2}{{\left( {N{H_3}} \right)}_4}} \right]Cl$$
C
$$\left[ {CoC{l_3}{{\left( {N{H_3}} \right)}_3}} \right]$$
D
$$\left[ {CoCl{{\left( {N{H_3}} \right)}_5}} \right]C{l_2}$$
Answer :
$$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]C{l_3}$$
342.
Which one of the following complexes will most likely absorb visible light ?
$${\text{(At nos}}{\text{. }}Sc = 21,Ti = 22,V = 23,Zn = 30\,)$$
A
$${\left[ {Sc{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$$
B
$${\left[ {Ti{{\left( {N{H_3}} \right)}_6}} \right]^{4 + }}$$
C
$${\left[ {V{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
D
$${\left[ {Zn{{\left( {N{H_3}} \right)}_6}} \right]^{2 + }}$$
Answer :
$${\left[ {V{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
343.
Mark the correct labelling of different terms used in coordination compounds :
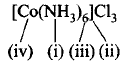
A
(i) Central atom, (ii) Ionisation sphere, (iii) Coordination number, (iv) Ligands
B
(i) Ligands, (ii) Coordination number, (iii) Valency, (iv) Ionisation sphere
C
(i) Ionisation sphere, (ii) Ligands, (iii) Coordination number, (iv) Central atom
D
(i) Ligands, (ii) Ionisation sphere, (iii) Coordination number, (iv) Central atom
Answer :
(i) Ligands, (ii) Ionisation sphere, (iii) Coordination number, (iv) Central atom
344.
Coordination number of $$Cr$$ is six. A complex with $${C_2}O_4^{2 - },en$$ and superoxide $$O_2^ - $$ will be in the ratio to make complex $${\left[ {Cr{{\left( {{C_2}{O_4}} \right)}_x},{{\left( {en} \right)}_y}{{\left( {{O_2}} \right)}_z}} \right]^ - }$$
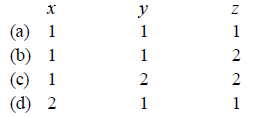
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(d)
345.
Three arrangements are shown for the complex, $${\left[ {Co\left( {en} \right){{\left( {N{H_3}} \right)}_2}B{r_2}} \right]^ + }.$$ Which one is the wrong statement?
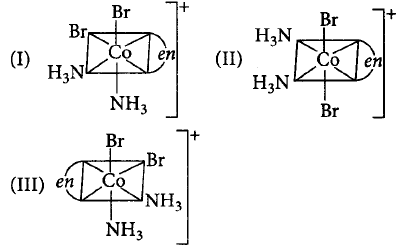
A
I and II are geometrical isomers.
B
II and III are optical isomers.
C
I and III are optical isomers.
D
II and III are geometrical isomers.
Answer :
II and III are optical isomers.
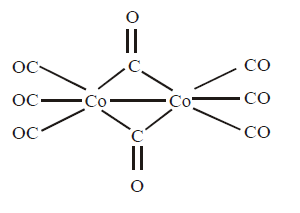
346. For $$\left[ {C{o_2}{{\left( {CO} \right)}_8}} \right],$$ what is the total number of metal - carbon bonds and number of metal-metal bonds.
A
10 ,1
B
8, 2
C
8, 1
D
10, 0
Answer :
10 ,1
347.
Which one of the following complexes is an outer orbital complex ?
$${\text{(Atomic nos}}{\text{. : }}Mn = 25;Fe = 26;Co = 27,Ni = 28\,)$$
A
$${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
B
$${\left[ {Mn{{\left( {CN} \right)}_6}} \right]^{4 - }}$$
C
$${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}$$
D
$${\left[ {Ni{{\left( {N{H_3}} \right)}_6}} \right]^{2 + }}$$
Answer :
$${\left[ {Ni{{\left( {N{H_3}} \right)}_6}} \right]^{2 + }}$$
348. Select the complex that can be reduced most easily.
A
$$Ni{\left( {CO} \right)_4}$$
B
$$Cr{\left( {CO} \right)_6}$$
C
$$V{\left( {CO} \right)_6}$$
D
$$Fe{\left( {CO} \right)_5}$$
Answer :
$$V{\left( {CO} \right)_6}$$
349. Which is not $$\pi - $$ bonded complex ?
A
Zeise’s salt
B
Ferrocene
C
Dibenzene chromiun
D
Tetraethyl lead
Answer :
Tetraethyl lead
350. Which of the following organometallic compounds is $$\sigma $$ and $$\pi $$ - bonded ?
A
$$\left[ {Fe{{\left( {{\eta ^5} - {C_5}{H_5}} \right)}_2}} \right]$$
B
$$K\left[ {PtC{l_3}\left( {{\eta ^2} - {C_2}{H_4}} \right)} \right]$$
C
$${\left[ {Co{{\left( {CO} \right)}_5}N{H_3}} \right]^{2 + }}$$
D
$$Fe{\left( {C{H_3}} \right)_3}$$
Answer :
$${\left[ {Co{{\left( {CO} \right)}_5}N{H_3}} \right]^{2 + }}$$